��Ŀ����
����Ŀ��Co��Ni�Ļ��������������������Ź㷺��Ӧ�á�
(1)![]() Ԫ�������ڱ��е�λ����4���ڣ�_________�塣��̬Coԭ�ӵĵ����Ų�ʽΪ____________��
Ԫ�������ڱ��е�λ����4���ڣ�_________�塣��̬Coԭ�ӵĵ����Ų�ʽΪ____________��
(2)�Ҷ���NH2-CH2-CH2-NH2����д����en����Nԭ�ӵ��ӻ���ʽΪ________�ӻ���en������Co�γ������[Co(en)2Cl2]Cl��HCl��2H2O�������ӽṹ����ͼ��ʾ���������ӵ���λ��Ϊ________������ᄃ���п��ܴ��ڵ���������___________��
A�����Ӽ� B�����Թ��ۼ� C���Ǽ��Թ��ۼ� D����λ�� E�����

(3)NiO����ṹ��ͼ1��ʾ�����������������AΪ(0,0,0)��BΪ(![]() )����C���ӣ����Ĵ����������Ϊ_______________��
)����C���ӣ����Ĵ����������Ϊ_______________��
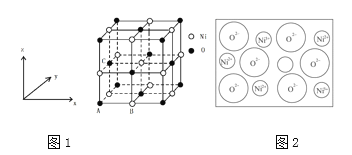
(4)��Ȼ���������������Ǵ��ھ���ȱ�ݣ���ͼ2��ʾ��NiXO������xֵΪ![]() ���������е�Ni�ֱ�Ϊ
���������е�Ni�ֱ�Ϊ![]() ��
��![]() ���˾�����
���˾�����![]() ��
��![]() �����������Ϊ______��
�����������Ϊ______��
���𰸡�VIII [Ar]3d74s2 sp3 6 ABCDE ��0��1/2��1/2�� 8:3
��������
��1��COԭ�ӵĺ˵����Ϊ27��λ��Ԫ�����ڱ��ĵ�4���ڵ�VIII�壬��̬CO�ĵ����Ų�ʽΪ[Ar]3d74s2��
��2���Ҷ�����H2N-CH2-CH2-NH2����Nԭ�Ӳ�ȡsp3�ӻ��������Ҷ�������������������Ϊ��λ���N������һ���Ҷ��������ṩ������λ����һ������λ������2��2+2=6���������Ļ�ѧʽ��֪��[Co(en)2Cl2]Cl��HCl��2H2O�����������Ҷ������Ӽ仯ѧ������λ����[Co(en)2Cl2]+��Cl-��Ļ�ѧ��Ϊ���Ӽ���H2O�������Ϊ����Լ����Թ��ۼ����Ҷ��������к��зǼ��Թ��ۼ�������ѡ��ABCDE��
��3����NiO����ṹ���Եó�C���ӵ����������
��4�������ӻ�����������������������������������������ĸ����������Ƚ��м��㡣
��1��COԭ�ӵĺ˵����Ϊ27��λ��Ԫ�����ڱ��ĵ�4���ڵ�VIII�壬��̬CO�ĵ����Ų�ʽΪ[Ar]3d74s2���ʴ�Ϊ��VIII��[Ar]3d74s2��
��2���Ҷ�����H2N-CH2-CH2-NH2����Nԭ�ӳ�3������������1�Թ¶Ե��ӣ��ӻ������Ϊ4����ȡsp3�ӻ��������Ҷ�������������������Ϊ��λ���N������һ���Ҷ��������ṩ������λ����һ������λ������2��2+2=6���������Ļ�ѧʽ��֪��[Co(en)2Cl2]Cl��HCl��2H2O�����������Ҷ������Ӽ仯ѧ������λ����[Co(en)2Cl2]+��Cl-��Ļ�ѧ��Ϊ���Ӽ���H2O�������Ϊ����Լ����Թ��ۼ����Ҷ��������к��зǼ��Թ��ۼ�������ѡ��ABCDE���ʴ�Ϊ��sp3��6��ABCDE��
��3����NiO�����������������AΪ��0��0��0����BΪ��1/2��0��0��������ͼ�ɿ���C�����������Ϊ��0��1/2��1/2�����ʴ�Ϊ����0��1/2��1/2����
��4��NiXO������xֵΪ0.88����Ni3+������Ϊx����Ni2+������Ϊ88-x���������ӻ�����������������������������������������ĸ����������ȿ�֪��3��x+��88-x����2=100��2����֮�ã�x=24������Ni2+��Ni3+��������֮�ȣ�88-24����24=8��3���ʴ�Ϊ��8��3��

����Ŀ����֪X��Y������Ԫ�أ�IΪ�����ܣ���λ��![]() ����������������жϣ��������
����������������жϣ��������
Ԫ�� |
|
|
|
|
X | 496 | 4562 | 6912 | 9543 |
Y | 578 | 1817 | 2745 | 11575 |
A. Ԫ��X�ij������ϼ���![]() ��
��
B. Ԫ��Y�Ǣ�A��Ԫ��
C. X���ʵ��۵����Y���ʵ��۵�
D. ��Ԫ��X���ڵ�3���ڣ����ĵ��ʿ�����ˮ���ҷ�Ӧ