��Ŀ����
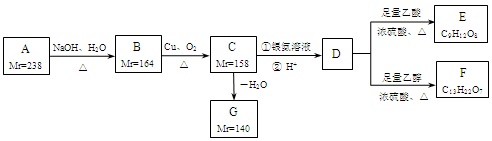
A��B��C��D��E�����л�����Ƿ�����C��H��O����Ԫ�ص������ȶ���6��1��8����ͨ��״���£�A��һ���д̼�����ζ�����壬������������ܶ�Ϊ15����ˮ��Һ�ܷ���������Ӧ��B����Է���������A��6����C��B��ͬ���칹�壬�����ʶ��Ǿ�����ζ����ɫ���壬��B�������ƾ���ҵ�Ļ�ԭ����D��E�����ʵ������ܶȶ���2.68 g��L��1(��״����)������Ҳ��Ϊͬ���칹�塣��D��ˮ��Һ��ʹʯ����Һ��죬��E�Dz�����ˮ����״Һ�壬����ˮ����ζ����д��A��B��C��D��E�����ƺͽṹ��ʽ��
A________��________��
B________��________��
C________��________��
D________��________��
E________��________��
��ȩ��HCHO
�����ǡ�CH2OH(CHOH)4CHO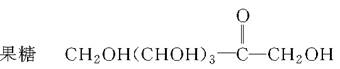
���ᡡCH3COOH
���������HCOOCH3
����

 ��У����ϵ�д�
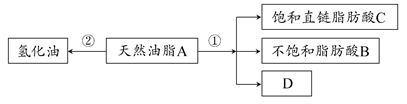
��У����ϵ�д����ࡢ֬���͵�����������������������Ӫ�����ʣ�����������ȷ���ǣ� ��
| A��ֲ���Ͳ��ܷ���ˮ�ⷴӦ | B���������ܷ���������Ӧ��ˮ�ⷴӦ |
| C������ˮ������ղ����������� | D��������ˮ������ղ������Ϊ������ |
�Ҵ����������̿�����ͼ��ʾ��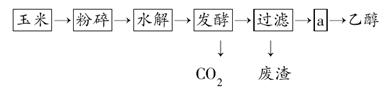
(1)��������Ŀ����___________________________��
(2)����������Ϊ�˼�������Ƿ���ȫˮ�⣬��ʹ�õ��Լ���________��
(3)����a�IJ����� (����)
| A������ | B����ȡ | C������ | D����Һ |
______________________________________________��
(5)�����ȵ���ԭ�������Ҵ��Ļ�ѧ��Ӧ������ʽ��ʾ��
(C6H10O5)n��nH2O
 nC6H12O6
nC6H12O6C6H12O6
 2C2H5OH��2CO2��
2C2H5OH��2CO2������������Ӧʽ���������100kg���������Ͽ�������ˮ�Ҵ�________kg��