��Ŀ����
��14�֣�
A��B��C��D�����ֳ������ʣ����ӦԪ�ص�ԭ������������������B��D���ڳ��������������Ϊ���������J��һ�ֺ�ɫ���壬I��Ũ��Һ���л�ԭ�ԣ�A��J����������֮�������µ�ת����ϵ�����ַ�Ӧ����ʡ�ԣ���

��1��BԪ�غ�CԪ�صļ����Ӱ뾶��С��ϵ�� �������ӷ��ű�ʾ���� _��
��2������Ԫ����C�γɵĻ�����NC3����ˮ�в���ʹ��ɫʯ����ֽ���������壬д���÷�Ӧ�Ļ�ѧ����ʽ ��
��3����E�ı�����Һ�����ˮ���γ���Һ�壬�ٽ���Һ��װ��U�ܣ�����U�ܵ����˲���缫����ֱͨ���磬�������˿ɹ۲쵽��������______________ ___��
��4��������J�����ữ��H2O2����Һ�У�J�ܽ���������+2�����ӣ��÷�Ӧ�����ӷ���ʽ��__________________________________________________ _��
��5������0��1 mol G����Һ�еμ�5 mol/L ��������Һ���õ�����3��9 g �������������������Ϊ______________________________��mL����
��1��r��Al3+�� < r��Cl‑������Al3+ < Cl‑�� ��2�֣�
��2��NCl3+ 3H2O = NH3�� + 3HClO ��3�֣�
��3�����ɫ��dz������Cl2���ɿɸ�1�֣���2�֣�
��4��MnO2 + H2O2 + 2H+ = Mn2+ + O2�� + 2H2O ��3�֣�
��5��10��50����2�֣�
��������

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�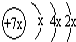 ��b��c�γɻ�����ĵ���ʽΪ
��b��c�γɻ�����ĵ���ʽΪ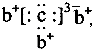 ���бȽ�����ȷ���ǣ�������
���бȽ�����ȷ���ǣ�������| A��ԭ�Ӱ뾶��a��c��d��b | B����ۺ����������c��d��a | C��ԭ��������a��d��b��c | D�����ʵ�������a��b��d��c |
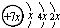 ��B��ͬ���ڵ�һ��������С��Ԫ�أ�C��������������ɵ����ӣ�E����Χ�����Ų�ʽΪ3d64s2���ش��������⣺
��B��ͬ���ڵ�һ��������С��Ԫ�أ�C��������������ɵ����ӣ�E����Χ�����Ų�ʽΪ3d64s2���ش��������⣺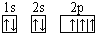
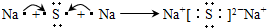
 ��B��ͬ���ڳ�ϡ��������뾶����Ԫ�أ�C��������� �����ɵ����ӣ�E��F����Χ�����Ų�ʽ�ֱ�Ϊ3d54s1��3d64s2���ش��������⣺
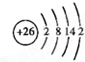
��B��ͬ���ڳ�ϡ��������뾶����Ԫ�أ�C��������� �����ɵ����ӣ�E��F����Χ�����Ų�ʽ�ֱ�Ϊ3d54s1��3d64s2���ش��������⣺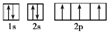
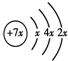
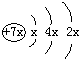 a��b��c��d�����ֶ�����Ԫ�أ�a��b��dͬ���ڣ�c��dͬ���壮a��ԭ�ӽṹʾ��ͼΪ��b��c�γɵĻ�����Ϊ b3c������b�Ļ��ϼ�Ϊ+1�����бȽ�����ȷ���ǣ�������
a��b��c��d�����ֶ�����Ԫ�أ�a��b��dͬ���ڣ�c��dͬ���壮a��ԭ�ӽṹʾ��ͼΪ��b��c�γɵĻ�����Ϊ b3c������b�Ļ��ϼ�Ϊ+1�����бȽ�����ȷ���ǣ�������