��Ŀ����
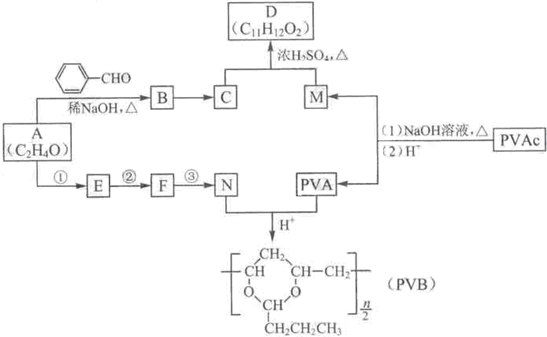
�����������ӵ��㾫�Ķ����D�Լ���������ȫ�����в�ĸ߷��ӻ�����PVB�ĺϳ�·�����£�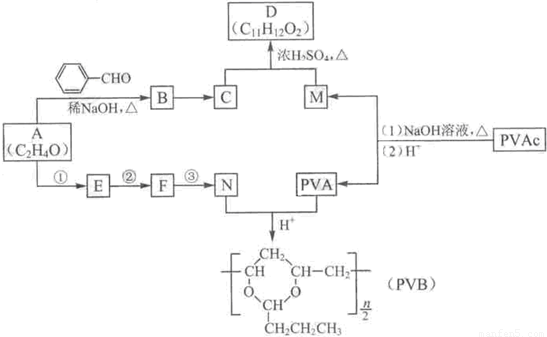
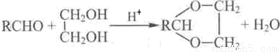
��֪����RCHO+R��CH2CHO
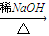
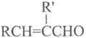 +H2O��R��R����ʾ�������⣩
+H2O��R��R����ʾ�������⣩��ȩ���Ԫ�����磺�Ҷ����������ɻ�״��ȩ��
��1��A�ĺ˴Ź������������ַ壮A��������______��
��2��A��
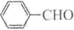 �ϳ�B�Ļ�ѧ����ʽ��______��
�ϳ�B�Ļ�ѧ����ʽ��______����3��CΪ��ʽ�ṹ����B��ԭ�õ���C�Ľṹʽ��______��
��4��E��ʹBr2��CCl4��Һ��ɫ��N��A����Ӧ�١��ۺϳɣ�
a���ٵķ�Ӧ�Լ���������______��
b���ڵķ�Ӧ������______��
c���۵Ļ�ѧ����ʽ��______��
��5��PVAc��һ�ֵ��徭�Ӿ۷�Ӧ�õ����õ���Ľṹ��ʽ��______��
��6�����������£�PVAc��ȫˮ��Ļ�ѧ����ʽ��______��
���𰸡�����������A�ķ���ʽ��Aת��ΪB�ķ�Ӧ��������������ϢI��A�ĺ˴Ź������ף�����ȷ��AΪ��ȩ��BΪ 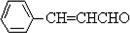 ����CΪ��ʽ�ṹ����B��ԭ�õ�������ȷ��C���Ծ���C=C������ԭ�Ļ���Ӧ��-CHO���ɴ˼���ȷ��C�Ľṹʽ
����CΪ��ʽ�ṹ����B��ԭ�õ�������ȷ��C���Ծ���C=C������ԭ�Ļ���Ӧ��-CHO���ɴ˼���ȷ��C�Ľṹʽ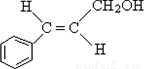 ������D�ķ���ʽC11H12O2������ȷ���䲻���Ͷ�Ϊ6���������D�ķ�Ӧ��������ȷ��DΪ
������D�ķ���ʽC11H12O2������ȷ���䲻���Ͷ�Ϊ6���������D�ķ�Ӧ��������ȷ��DΪ 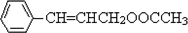 ������ȷ��MΪ�����PVB�Ľṹ��ʽ�������ϢII��ȷ���䵥��֮һ��CH3��CH2��2CHO����NΪCH3��CH2��2CHO����һ�߷��ӻ�����PVAΪ
������ȷ��MΪ�����PVB�Ľṹ��ʽ�������ϢII��ȷ���䵥��֮һ��CH3��CH2��2CHO����NΪCH3��CH2��2CHO����һ�߷��ӻ�����PVAΪ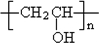 �������Ƴ�PVAc�ĵ���ΪCH3COOCH=CH2����A��N�Ľṹ��ʽ����������ϢI�����Ƴ�EΪ��CH3CH=CHCHO��FΪCH3��CH2��3OH���ɴ˼��ɰ�����Ҫ��ش��й����⣮
�������Ƴ�PVAc�ĵ���ΪCH3COOCH=CH2����A��N�Ľṹ��ʽ����������ϢI�����Ƴ�EΪ��CH3CH=CHCHO��FΪCH3��CH2��3OH���ɴ˼��ɰ�����Ҫ��ش��й����⣮
����⣺��1��A�ķ���ʽ��C2H4O����A�ĺ˴Ź������������ַ壬���Aֻ������ȩ���ʴ�Ϊ����ȩ��
��2��AΪ��ȩ��BΪ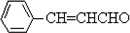 ����Ӧ�ķ���ʽΪ
����Ӧ�ķ���ʽΪ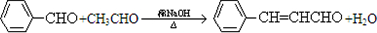 ��
��
�ʴ�Ϊ��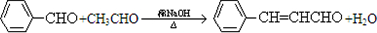 ��
��
��3��CΪ��ʽ�ṹ��˵��C�к���̼̼˫��������ΪC��B��ԭ�õ���B�к���ȩ�������C�к����ǻ�����C�Ľṹ��ʽ��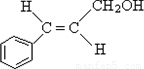 ��
��
�ʴ�Ϊ��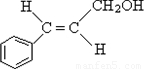 ��
��
��4������PVB�Ľṹ��ʽ�������Ϣ����Ƴ�N�Ľṹ��ʽ��CH3��CH2��2CHO������ΪE��ʹBr2��CCl4��Һ��ɫ������E��2������ȩ������������Һ�в����ȵ����������ɵģ���E�Ľṹ��ʽ��CH3CH=CHCHO��Ȼ��Eͨ�������ӳɵõ�F������F�Ľṹ��ʽ��CH3CH2CH2CH2OH��F�����������õ�N������ʽΪ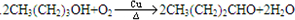 ��
��
�ʴ�Ϊ��aϡNaOH�����ȣ�b�ӳɷ�Ӧ��c��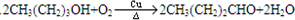 ��
��
��5����C��D�Ľṹ��ʽ��֪M�����ᣬ��PVB��N�Ľṹ��ʽ��֪PVA�Ľṹ��ʽ�Ǿ���ϩ�������PVAC�ĵ�����������ϩ�����ṹ��ʽ��CH3COOCH=CH2��
�ʴ�Ϊ��CH3COOCH=CH2��
��6��PVAc�ĵ���ΪCH3COOCH=CH2����PVAcΪ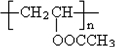 ���������������ڼ���������ˮ������
���������������ڼ���������ˮ������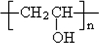 �������ƣ���Ӧ�Ļ�ѧ����ʽΪ
�������ƣ���Ӧ�Ļ�ѧ����ʽΪ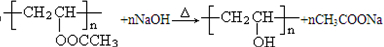 ��
��
�ʴ�Ϊ��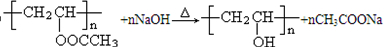 ��
��
���������⿼���л���ĺϳɣ���Ŀ�ѶȽϴ����ʱע��������е�������Ϣ�����С����е���Ϣ��Ϊ�����Ĺؼ���ע������л�������ŵĽṹ�����ʣ�
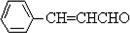 ����CΪ��ʽ�ṹ����B��ԭ�õ�������ȷ��C���Ծ���C=C������ԭ�Ļ���Ӧ��-CHO���ɴ˼���ȷ��C�Ľṹʽ
����CΪ��ʽ�ṹ����B��ԭ�õ�������ȷ��C���Ծ���C=C������ԭ�Ļ���Ӧ��-CHO���ɴ˼���ȷ��C�Ľṹʽ ������D�ķ���ʽC11H12O2������ȷ���䲻���Ͷ�Ϊ6���������D�ķ�Ӧ��������ȷ��DΪ
������D�ķ���ʽC11H12O2������ȷ���䲻���Ͷ�Ϊ6���������D�ķ�Ӧ��������ȷ��DΪ 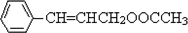 ������ȷ��MΪ�����PVB�Ľṹ��ʽ�������ϢII��ȷ���䵥��֮һ��CH3��CH2��2CHO����NΪCH3��CH2��2CHO����һ�߷��ӻ�����PVAΪ
������ȷ��MΪ�����PVB�Ľṹ��ʽ�������ϢII��ȷ���䵥��֮һ��CH3��CH2��2CHO����NΪCH3��CH2��2CHO����һ�߷��ӻ�����PVAΪ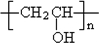 �������Ƴ�PVAc�ĵ���ΪCH3COOCH=CH2����A��N�Ľṹ��ʽ����������ϢI�����Ƴ�EΪ��CH3CH=CHCHO��FΪCH3��CH2��3OH���ɴ˼��ɰ�����Ҫ��ش��й����⣮
�������Ƴ�PVAc�ĵ���ΪCH3COOCH=CH2����A��N�Ľṹ��ʽ����������ϢI�����Ƴ�EΪ��CH3CH=CHCHO��FΪCH3��CH2��3OH���ɴ˼��ɰ�����Ҫ��ش��й����⣮����⣺��1��A�ķ���ʽ��C2H4O����A�ĺ˴Ź������������ַ壬���Aֻ������ȩ���ʴ�Ϊ����ȩ��
��2��AΪ��ȩ��BΪ
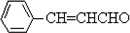 ����Ӧ�ķ���ʽΪ
����Ӧ�ķ���ʽΪ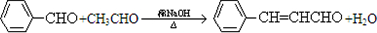 ��
���ʴ�Ϊ��
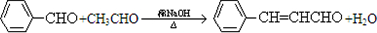 ��
����3��CΪ��ʽ�ṹ��˵��C�к���̼̼˫��������ΪC��B��ԭ�õ���B�к���ȩ�������C�к����ǻ�����C�Ľṹ��ʽ��
 ��
���ʴ�Ϊ��
 ��
����4������PVB�Ľṹ��ʽ�������Ϣ����Ƴ�N�Ľṹ��ʽ��CH3��CH2��2CHO������ΪE��ʹBr2��CCl4��Һ��ɫ������E��2������ȩ������������Һ�в����ȵ����������ɵģ���E�Ľṹ��ʽ��CH3CH=CHCHO��Ȼ��Eͨ�������ӳɵõ�F������F�Ľṹ��ʽ��CH3CH2CH2CH2OH��F�����������õ�N������ʽΪ
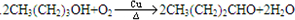 ��
���ʴ�Ϊ��aϡNaOH�����ȣ�b�ӳɷ�Ӧ��c��
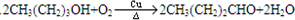 ��
����5����C��D�Ľṹ��ʽ��֪M�����ᣬ��PVB��N�Ľṹ��ʽ��֪PVA�Ľṹ��ʽ�Ǿ���ϩ�������PVAC�ĵ�����������ϩ�����ṹ��ʽ��CH3COOCH=CH2��
�ʴ�Ϊ��CH3COOCH=CH2��
��6��PVAc�ĵ���ΪCH3COOCH=CH2����PVAcΪ
 ���������������ڼ���������ˮ������
���������������ڼ���������ˮ������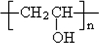 �������ƣ���Ӧ�Ļ�ѧ����ʽΪ
�������ƣ���Ӧ�Ļ�ѧ����ʽΪ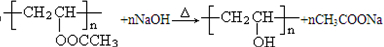 ��
���ʴ�Ϊ��
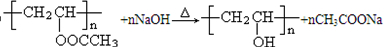 ��
�����������⿼���л���ĺϳɣ���Ŀ�ѶȽϴ����ʱע��������е�������Ϣ�����С����е���Ϣ��Ϊ�����Ĺؼ���ע������л�������ŵĽṹ�����ʣ�

��ϰ��ϵ�д�
 �������ϵ�д�
�������ϵ�д�
�����Ŀ
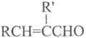 +H2O��R��R����ʾ�������⣩
+H2O��R��R����ʾ�������⣩
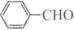 �ϳ�B�Ļ�ѧ����ʽ��
�ϳ�B�Ļ�ѧ����ʽ��








 ��R��R�@��ʾ�������⣩
��R��R�@��ʾ�������⣩
 �ϳ�B�Ļ�ѧ����ʽ��
�ϳ�B�Ļ�ѧ����ʽ��
 ��R��R���ʾ������
��R��R���ʾ������
 �ϳ�B�Ļ�ѧ����ʽ��
�ϳ�B�Ļ�ѧ����ʽ��


 �ϳ�B�Ļ�ѧ����ʽ��
�ϳ�B�Ļ�ѧ����ʽ��  ��
�� ��Һ��ɫ��N��A����Ӧ1~3�ϳɡ�
��Һ��ɫ��N��A����Ӧ1~3�ϳɡ�