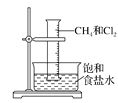
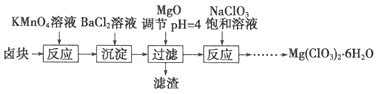
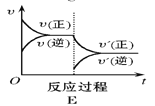
ЬтФПФкШн
ЁОЬтФПЁПSO2 КЭ NOx ЖМЪЧДѓЦјЮлШОЮяЁЃ
(1)РћгУАБЫЎПЩвдНЋ SO2 КЭ NO2 ЮќЪеЃЌдРэШчЯТЭМЫљЪОЃК

NO2 БЛЮќЪеЕФРызгЗНГЬЪНЪЧ__________________ЁЃ
(2)ЪЊЗЈЮќЪеЙЄвЕЮВЦјжаЕФNO2ЃЌГЃбЁгУДПМюШмвКЃЌНЋNO2зЊЛЏЮЊСНжжЕЊдЊЫиЕФГЃМћКЌбѕЫсбЮЃЌЦфЗДгІЕФРызгЗНГЬЪНЪЧ(вбжЊЫсадHNO2ЃОH2CO3)______________ЁЃ
(3)гУИпФмЕчзгЪјМЄЛюбЬЦј(жївЊГЩЗжЪЧSO2ЁЂNO2ЁЂH2OЕШ)ЃЌЛсВњЩњO3ЕШЧПбѕЛЏадЮЂСЃЁЃбЬЦјОЯТСаЙ§ГЬПЩЛёЕУЛЏЗЪЁЃ

ИУЛЏЗЪжаКЌгаЕФРызгЪЧ![]() ЁЂ___________(ЬюРызгЗћКХ)ЁЃ
ЁЂ___________(ЬюРызгЗћКХ)ЁЃ
(4)SCR КЭ NSR ММЪѕПЩгааЇНЕЕЭВёгЭЗЂЖЏЛњдкПеЦјЙ§СПЬѕМўЯТЕФ NOx ХХЗХЁЃ
IЃЎSCR(бЁдёадДпЛЏЛЙд)ЙЄзїдРэЃК

ЂйФђЫи[CO(NH2)2]ЫЎШмвКШШЗжНтЮЊ NH3 КЭ CO2ЃЌИУЗДгІЕФЛЏбЇЗНГЬЪНЪЧ________________ЁЃ
ЂкЗДгІЦїжа NH3 ЛЙд NO2 ЕФЛЏбЇЗНГЬЪНЪЧ_________________ЁЃ
ЂлЕБШМгЭжаКЌСђСПНЯИпЪБЃЌЮВЦјжа SO2 дк O2 зїгУЯТЛсаЮГЩ(NH4)2SO4ЃЌЪЙДпЛЏМСжаЖОЁЃгУЛЏбЇЗНГЬЪНБэЪО(NH4)2SO4 ЕФаЮГЩ_____________________ЁЃ
IIЃЎNSR(NOx ДЂДцЛЙд)ЙЄзїдРэЃКNOx ЕФДЂДцКЭЛЙддкВЛЭЌЪБЖЮНЛЬцНјааЃЌШчЯТЭМЫљЪОЁЃ

ЂйЭЈЙ§ BaO КЭ Ba(NO3)2 ЕФЯрЛЅзЊЛЏЪЕЯж NOx ЕФДЂДцКЭЛЙдЁЃДЂДц NOx ЕФЮяжЪЪЧ___________________ЃЌЩњГЩ Ba(NO3)2 ЕФЛЏбЇЗНГЬЪНЪЧ _______________ЁЃ
ЂкгУ H2 ФЃФтЮВЦјжаЛЙдадЦјЬхбаОПСЫ Ba(NO3)2 ЕФДпЛЏЛЙдЙ§ГЬЃЌИУЙ§ГЬЗжСНВННјааЃЌЯТЭМБэЪОИУЙ§ГЬЯрЙиЮяжЪХЈЖШЫцЪБМфЕФБфЛЏЙиЯЕЁЃЕквЛВНЗДгІЯћКФЕФH2 гы Ba(NO3)2 ЕФЮяжЪЕФСПжЎБШЪЧ___________ЁЃ

ЂлЛЙдЙ§ГЬжаЃЌгаЪБЛсВњЩњаІЦј(N2O)ЁЃгУЭЌЮЛЫиЪОзйЗЈбаОПЗЂЯжаІЦјЕФВњЩњгы NO гаЙиЁЃдкгабѕЦјЬѕМўЯТ 15NO гы NH3 вдвЛЖЈБШР§ЗДгІЪБЃЌЕУЕНЕФаІЦјМИКѕЖМЪЧ 15N1.5N0.5OЁЃНЋИУЗДгІЕФЛЏбЇЗНГЬЪНВЙГфЭъећЃК_____________ ![]() _________15N1.5N0.5O+________H2O
_________15N1.5N0.5O+________H2O
ЁОД№АИЁП2NO2+4![]() =N2+4
=N2+4![]() +4H+
+4H+ ![]() +2NO2=
+2NO2=![]() +
+![]() +CO2
+CO2 ![]() ЁЂ
ЁЂ![]() CO(NH2)2+H2O
CO(NH2)2+H2O![]() 2NH3Ёќ+CO2Ёќ 8NH3+6NO2
2NH3Ёќ+CO2Ёќ 8NH3+6NO2![]() 7N2+12H2O 2SO2+O2+4NH3+2H2OЈT2(NH4)2SO4 BaO 2BaO+4NO+3O2=2Ba(NO3)2 8:1 1215NO+4NH3+O2 8 6
7N2+12H2O 2SO2+O2+4NH3+2H2OЈT2(NH4)2SO4 BaO 2BaO+4NO+3O2=2Ba(NO3)2 8:1 1215NO+4NH3+O2 8 6
ЁОНтЮіЁП
(1) ИљОнСїГЬЭМаХЯЂNO2БЛЮќЪеЪБЗДгІЮяЮЊNO2ЁЂNH4HSO3ЃЌЩњГЩЮягаЕЊЦјЃЌОнДЫвРОнбѕЛЏЛЙдЗДгІЕФЕУЪЇЕчзгЪиКуЁЂдзгЪиКуКЭЕчКЩЪиКуЪщаДРызгЗДгІЗНГЬЪНЃЛ
(2) ДПМюШмвКЃЌНЋNO2зЊЛЏЮЊСНжжЕЊдЊЫиЕФГЃМћКЌбѕЫсбЮЃЌНсКЯдзгЪиКуКЭбѕЛЏЛЙдЗДгІЕчзгЪиКуЗжЮіЗДгІЩњГЩЯѕЫсбЮКЭбЧЯѕЫсбЮЃЛ
(3) гУИпФмЕчзгЪјМЄЛюбЬЦј(жївЊГЩЗжЪЧSO2ЁЂNO2ЁЂH2OЕШ)ЃЌЛсВњЩњO3ЕШЧПбѕЛЏадЮЂСЃЃЌO3гыбЬЦјжаЕФSO2ЁЂNO2ЁЂH2OЗДгІЩњГЩЕФH2SO4КЭHNO3гызЂШыЕФNH3ЗДгІЃЌЩњГЩЛЏЗЪСђяЇКЭЯѕяЇЃЛ
(4) IЃЎЯШХаЖЯЗДгІЮяКЭЩњГЩЮяЃЌдйНсКЯЕчзгЪиКуКЭдзгЪиКуЪщаДгаЙиЗДгІЗНГЬЪНЃЛ
IIЃЎЂйгЩЭМПЩжЊДЂДцNOxЕФЮяжЪЪЧBaOЃЛ
ЂкЕквЛВНЗДгІжаH2БЛбѕЛЏЩњГЩЫЎЃЌЧтдЊЫиЛЏКЯМлгЩ0МлЩ§ИпЕН+1МлЃЌBa(NO3)2ЕФNдЊЫиЛЏКЯМлгЩ+5МлНЕЕЭЕН-3МлЃЌЩњГЩАБЦјЃЌНсКЯЕУЪЇЕчзгЪ§ФПЯрЕШМЦЫуЃЛ
ЂлдкгабѕЬѕМўЯТ15NOгыNH3вдвЛЖЈБШР§ЗДгІЪБЃЌЕУЕНЕФаІЦјМИКѕЖМЪЧ15N1.5N0.5OЃЌгЩNдЊЫиЪиКуПЩжЊ15NOгыNH3гІЮЊ3ЃК1ЃЌНсКЯЕчзгЕУЪЇЯрЕШХфЦНЁЃ
(1) NO2БЛЮќЪеЪБЃЌЗДгІЮяЮЊNO2ЁЂNH4HSO3ЃЌЖўбѕЛЏЕЊжаЕЊдЊЫиЮЊ+4МлЃЌЩњГЩЮягаЕЊЦјЃЌЫљвдбЧСђЫсЧтИљРызгжаСђБЛбѕЛЏГЩСђЫсИљРызгЃЌИљОнЕУЪЇЕчзгЪиКуЁЂдзгЪиКуКЭЕчКЩЪиКуЃЌЗДгІЕФРызгЗНГЬЪНЮЊ2NO2+4![]() =N2+4
=N2+4![]() +4H+ЃЛ
+4H+ЃЛ
(2) ДПМюШмвКЃЌНЋNO2зЊЛЏЮЊСНжжЕЊдЊЫиЕФГЃМћКЌбѕЫсбЮЃЌНсКЯдзгЪиКуКЭбѕЛЏЛЙдЗДгІЕчзгЪиКуЗжЮіЗДгІЩњГЩЯѕЫсбЮКЭбЧЯѕЫсбЮЃЌЗДгІЕФРызгЗНГЬЪНЮЊЃК![]() +2NO2=
+2NO2=![]() +
+![]() +CO2ЃЛ
+CO2ЃЛ
(3) гУИпФмЕчзгЪјМЄЛюбЬЦј(жївЊГЩЗжЪЧSO2ЁЂNO2ЁЂH2OЕШ)ЃЌЛсВњЩњO3/span>ЕШЧПбѕЛЏадЮЂСЃЃЌO3гыбЬЦјжаЕФSO2ЁЂNO2ЁЂH2OЗДгІЩњГЩЕФH2SO4КЭHNO3гыМгШыЕФNH3ЗЂЩњШчЯТЗДгІЃКH2SO4+2NH3=(NH4)2SO4ЁЂHNO3+NH3 =NH4NO3ЃЌИУЛЏЗЪжаКЌгаЕФРызгЪЧ![]() ЁЂ
ЁЂ![]() ЁЂ
ЁЂ![]() ЃЛ
ЃЛ
(4) IЃЎЂйФђЫи[CO(NH2)2]ЫЎШмвКШШЗжНтЮЊNH3КЭCO2ЃЌЗДгІЮяЮЊФђЫиКЭЫЎЃЌЩњГЩЮяЮЊАБЦјКЭЖўбѕЛЏЬМЃЌИљОндзгЪиКуЃЌЗДгІЕФЛЏбЇЗНГЬЪНЮЊЃКCO(NH2)2+H2O![]() 2NH3Ёќ+CO2ЁќЃЛ
2NH3Ёќ+CO2ЁќЃЛ
ЂкNH3дкДпЛЏМСзїгУЯТЛЙдNO2ЩњГЩЕЊЦјКЭЫЎЃЌИљОнЕУЪЇЕчзгЪиКуКЭдзгЪиКуЃЌЗДгІЕФЛЏбЇЗНГЬЪНЮЊ8NH3+6NO2![]() 7N2+12H2OЃЛ
7N2+12H2OЃЛ
ЂлSO2дкO2зїгУЯТгыNH3ЁЂH2OЗДгІаЮГЩ(NH4)2SO4ЃЌДЫЗДгІжаSO2ЪЧЛЙдМСЃЌбѕЦјЪЧбѕЛЏМСЃЌИљОнЕУЪЇЕчзгЪиКуКЭдзгЪиКуЃЌЗДгІЕФЛЏбЇЗНГЬЪНЮЊ2SO2+O2+4NH3+2H2OЈT2(NH4)2SO4ЃЛ
IIЃЎЂйгЩЭМЪОПЩжЊBaOКЭNOxЗДгІЩњГЩBa(NO3)2ЃЌBa(NO3)2дйЛЙдЮЊN2ЃЌдђДЂДцNOxЕФЮяжЪЮЊBaOЃЛДЂДцЪБЃЌбѕЛЏБЕЁЂвЛбѕЛЏЕЊКЭбѕЦјЗДгІЩњГЩBa(NO3)2ЕФЛЏбЇЗНГЬЪНЪЧ2BaO+4NO+3O2=2Ba(NO3)2ЃЛ
ЂкЕквЛВНЗДгІжаH2БЛбѕЛЏЩњГЩЫЎЃЌHдЊЫиЕФЛЏКЯМлгЩ0МлЩ§ИпЕН+1МлЃЌBa(NO3)2ЕФNдЊЫиЛЏКЯМлгЩ+5МлНЕЕЭЕН-3МлЃЌЩњГЩАБЦјЃЌдђ1molBa(NO3)2ЩњГЩАБЦјЕУЕН16molЕчзгЃЌВЮМгЗДгІЕФЧтЦјЕФЮяжЪЕФСПЮЊ![]() mol=8molЃЌдђЯћКФЕФH2гыBa(NO3)2ЕФЮяжЪЕФСПжЎБШЪЧ8:1ЃЛ
mol=8molЃЌдђЯћКФЕФH2гыBa(NO3)2ЕФЮяжЪЕФСПжЎБШЪЧ8:1ЃЛ
ЂлдкгабѕЦјЬѕМўЯТ15NOгыNH3вдвЛЖЈБШР§ЗДгІЪБЃЌЕУЕНЕФаІЦјМИКѕЖМЪЧ15N1.5N0.5OЃЌгЩNдЊЫиЪиКуПЩжЊ15NOгыNH3гІЮЊ3ЃК1ЃЌ1mol15NOБЛЛЙдЕУЕН1molЕчзгЃЌШєга3mol15NOВЮгыЗДгІЃЌдђ3mol15NOБЛЛЙдЕУЕН3molЕчзгЃЌ1molNH3БЛбѕЛЏЪЇШЅ4molЕчзгЃЌИљОнЕчзгЪиКуЃЌВЮгыЗДгІЕФO2ЮЊ0.25molЃЌМД15NOЁЂNH3ЁЂO2ЮяжЪЕФСПжЎБШЮЊ3molЃК1molЃК0.25mol=12:4:1ЃЌПЩжЊЗДгІЕФЛЏбЇЗНГЬЪНЮЊ1215NO+4NH3+O2![]() 815N1.5N0.5O +6H2OЁЃ
815N1.5N0.5O +6H2OЁЃ

ЁОЬтФПЁПФГаЁзщЭЌбЇЮЊбаОП NaHCO3 ШмвКМгШШЪБЕФ pH БфЛЏМАЦфдвђЃЌдк 10ЁцЪБМгШШNaHCO3 ШмвКЃЌВЂМЧТМ pH ШчЯТЃК
ЮТЖШ(Ёц) | 10 | 20 | 30 | МгШШжѓЗаКѓРфШДЕН 50Ёц |
pH | 8.3 | 8.4 | 8.5 | 8.8 |
(1)МзЭЌбЇШЯЮЊЃЌИУШмвКЕФ pH Щ§ИпЕФдвђЪЧ![]() ЕФЫЎНтГЬЖШдіДѓЃЌЙЪМюаддіЧПЃЌNaHCO3ЗЂЩњЫЎНтЗДгІЕФРызгЗНГЬЪНЪЧ______ЁЃ
ЕФЫЎНтГЬЖШдіДѓЃЌЙЪМюаддіЧПЃЌNaHCO3ЗЂЩњЫЎНтЗДгІЕФРызгЗНГЬЪНЪЧ______ЁЃ
(2)ввЭЌбЇШЯЮЊЃЌШмвК pH Щ§ИпЕФдвђЪЧNaHCO3 ЪмШШЗжНтЃЌЩњГЩСЫ Na2CO3ЃЌВЂЭЦЖЯ Na2CO3ЕФЫЎНтГЬЖШ_______________(ЬюЁАЃОЁБЛђЁАЃМЁБ)NaHCO3ЁЃ
(3)БћЁЂЖЁЭЌбЇНјааЪЕбщШчЯТЃК
ЂйБћЃКЯђЩйСПМгШШжѓЗаЕФШмвККЭЮДОМгШШЕФШмвКжаЗжБ№МгШызуСПЕФЪдМС X КѓЃЌЙлВьЕНЧАепВњЩњДѓСПАзЩЋГСЕэЃЌвђДЫЫћШЯЮЊ______ (ЬюЁАМзЁБЛђЁАввЁБ)ЕФХаЖЯе§ШЗЁЃЦфжаЪдМС X ЪЧ________(ЬюађКХ)ЁЃ
aЃЎBa(OH)2 ШмвК b. BaCl2 ШмвК c. NaOH ШмвК d. ГЮЧхЪЏЛвЫЎ
ЂкЖЁЃКНЋМгШШжС 30ЁцЕФ NaHCO3 ШмвКЛжИДжС 10ЁцЃЌВтЦф pH=8.3ЁЃгЩДЫПЩЕУЕНЕФНсТлЪЧ__________ЁЃ