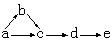��Ŀ����
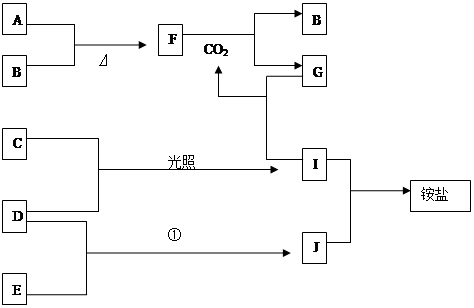
��13�֣���ͼ�Dz��ֶ�����Ԫ�صĵ��ʼ��仯���������Һ����ת����ϵ����֪B��C��D��E�Ƿǽ������ʣ����ڳ��³�ѹ�¶������壬������G����ɫ��ӦΪ��ɫ��������I��Jͨ��״���³���̬����Ӧ���ǻ��������е�һ����Ҫ�̵���Ӧ��
��ش��������⣺
��1��д���������ʻ�ѧʽ��A B C
(2)д���������ʼ䷴Ӧ�Ļ�ѧ����ʽ
A+B
F+CO2
D+E
I+J
(3)д�����з�Ӧ�����ӷ���ʽ
G+I

��ش��������⣺
��1��д���������ʻ�ѧʽ��A B C
(2)д���������ʼ䷴Ӧ�Ļ�ѧ����ʽ
A+B
F+CO2
D+E
I+J
(3)д�����з�Ӧ�����ӷ���ʽ
G+I
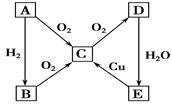
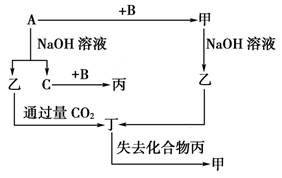
��1��Na ����Na2O�� O2 Cl2
��2�� 2Na + O2 ="=" Na2O2 �� 2Na2O + O2 == 2Na2O2
2Na2O2 + 2CO2 == 2Na2CO3 + O2 N2 + 3H2 == 2NH3
NH3 + HCl == NH4Cl CO32�� + 2H+ == CO2 �� + H2O
��2�� 2Na + O2 ="=" Na2O2 �� 2Na2O + O2 == 2Na2O2
2Na2O2 + 2CO2 == 2Na2CO3 + O2 N2 + 3H2 == 2NH3
NH3 + HCl == NH4Cl CO32�� + 2H+ == CO2 �� + H2O
����������ͼ�⣬�ؼ�����ͻ�Ƶ㡣��Ӧ���ǻ��������е�һ����Ҫ�̵���Ӧ����J�ǰ�����I���Ȼ��⡣����C��������D��������E�ǵ�����������G����ɫ��ӦΪ��ɫ��˵��������Ԫ�ء�����G���Ȼ��ⷴӦ����CO2����B�����壬����G��̼���ƣ� F�ǹ������ƣ�B����������A���ƻ������ơ�

��ϰ��ϵ�д�
�����Ŀ