��Ŀ����
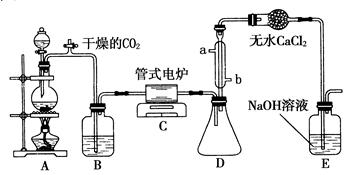
��ˮ�Ȼ����ǰ�ɫ���壬������ˮ�֣���178��������װ����ˮ�Ȼ���¶���ڳ�ʪ�����лᱬը������������������ҵ���ɽ������������û�����ˮ�Ȼ�������������Al���ö��Ƶã�ij����С����ʵ������ͨ������װ��(����ͼ)��ȡ������������ˮ�Ȼ�����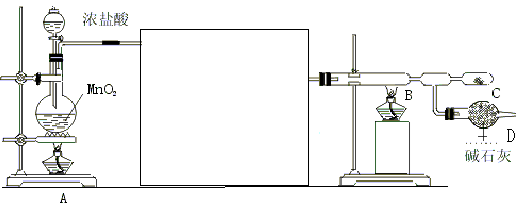
�Իش��������⣺
��1��װ��A�з�Ӧ�Ļ�ѧ����ʽ
��2��Ϊʹʵ��ɹ���A��B����Ҫ���ʵ���װ�ã��뽫�����ڿ��ڣ���ע������ʢ�ŵ�ҩƷ�����A����������ֱ�ӽ���B�У�ʵ������IJ�������� ��
��3������ʵ��ʱ��Ӧ�ȵ�ȼ����д��ĸ����ͬ�� ���ľƾ��ƣ�Ȼ���ٵ�ȼ
���ľƾ��ơ�
��4����C�������ռ����������Ȼ�����ԭ���� ��
��5��װ��D�������� ��
��1��MnO2��4HCl(Ũ)  MnCl2��Cl2����2H2O
MnCl2��Cl2����2H2O
��2�� AlCl3��H2O��Ӧ����ը����3��A �� B ��4�� AlCl3������
AlCl3��H2O��Ӧ����ը����3��A �� B ��4�� AlCl3������
��5�����ն����Cl2�ҷ�ֹˮ��������CʹAlCl3����ˮ��
���������������1��Ũ����Ͷ��������ڼ����������ܷ���������ԭ��Ӧ�����Ȼ��̡�������ˮ������װ��A�з�Ӧ�Ļ�ѧ����ʽΪMnO2��4HCl(Ũ)  MnCl2��Cl2����2H2O��
MnCl2��Cl2����2H2O��
��2��Ũ������лӷ��ԣ��������ɵ������к����Ȼ����ˮ�������Ȼ�������ܷ�Ӧ����������������������ϻᷢ����ը�������Ȼ���������ˮ����ˮ�Ȼ���¶���ڳ�ʪ�����лᱬը����������������������ͨ��B֮ǰ��Ҫ��ȥ�Ȼ����ˮ������ʹ�õ��Լ��ֱ��DZ���ʳ��ˮ��Ũ���ᡣ
��3������װ���л����п��������Ҫ���������������ų����Լ���ֹ�������������������Խ���ʵ��ʱ��Ӧ�ȵ�ȼA���ľƾ��ƣ�Ȼ���ٵ�ȼB���ľƾ��ơ�
��4������AlCl3��������������C�������ռ���������
��5�������ж�����Ⱦ������������Ҫβ����������һ�����Ȼ���������ˮ����˼�ʯ�ҵĵ���Ҫ�����ն����Cl2�ҷ�ֹˮ��������CʹAlCl3����ˮ�⡣
���㣺�����������Ʊ��������ľ�����β�������Լ���ѧʵ�����������
�����������Ǹ߿��еij�����������ͣ������е��Ѷ�����Ŀ��飬�����ۺ���ǿ�����ض�ѧ�������������ͽ��ⷽ����ָ����ѵ����ּ�ڿ���ѧ��������û���֪ʶ���ʵ�����������������������ѧ���������������淶�Ͻ���ʵ��������������ѧ����ѧ�����������������ۺ���ǿ�����ۺ�ʵ������ϵ���ܣ��еĻ��ṩһЩ�µ���Ϣ�����Ҫ��ѧ���������桢ϸ�µ����⣬��ϵ��ѧ����֪ʶ�ͼ��ܣ�����֪ʶ����ȡ�Ǩ�ơ����飬ȫ��ϸ�µ�˼�����ܵó���ȷ�Ľ��ۡ�

SO2��һ����Ҫ�Ļ���ԭ�ϣ�Ҳ������������Ⱦ����Ҫ��Դ��
(1)ij��ȤС�������ͼ��ʾװ����ȡSO2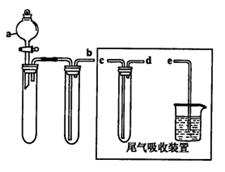
������ʵ�鷽��������ͼ��ʾװ����ȡ����SO2���Լ���_______(����ţ���
| A��Na2SO3��Һ��ϡ���� |
| B��Na2SO3������Ũ���� |
| C������������� |
| D��ͭ��Ũ���� |
��β������װ�õ�����˳����b��( )�� ( )��e��
(2)Ϊ�˻�������SO2�������о���Ա���������õ�Ʒλ���̿�(��Ҫ�ɷ���MnO2)���ո��±��պ������������SO2�������Ʊ������̾���(
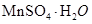 �������̣�������ʾ��ͼ���£�
�������̣�������ʾ��ͼ���£�
��֪������Һ��pH<2�����еĽ���������Ҫ��Mn2+��������������Fe2����Al3���������������ӡ��йؽ��������γ������������ʱ��Һ��pH���±���
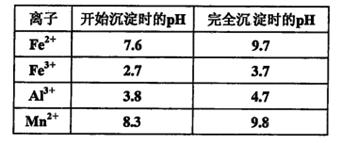
��ش�
�ٺ�Al3�����γ�������ˮ���������ӷ���ʽ��ʾ�侻ˮԭ��________________________��
�ڽ�����������Ҫ��Ӧ�Ļ�ѧ����ʽ��___________________________________________��
�����������м���MnO2�۵�Ŀ����______________________________________________;
��Ӧ�����ӷ���ʽ��_________________________________________________________��
����ʯ�ҽ�����pH��pHӦ���ڵķ�Χ��___________________________________��
����������Ҫ�ɷ���____________________________________��
ҽ���Ȼ��ƿ����ڲ��ơ������������ȣ��Թ�ҵ̼��ƣ�������Na+��Al3+��Fe3+�����ʣ�����ҽ�ö�ˮ���Ȼ��ƹ�������Ϊ�� 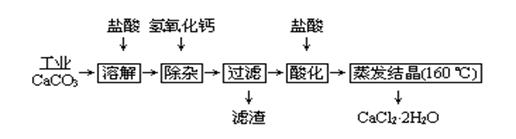
��֪���������ϵ�֪�����������ʱ��pHΪ��
| �������� | Fe(OH)3 | Al(OH)3 | |
| ��ʼ����ʱ��pH | 2.3 | 4.0 | ��ʼ�ܽ⣺7.8 |
| ��ȫ����ʱ��pH | 3.7 | 5.2 | ��ȫ�ܽ⣺10.8 |
��1��CaCO3�����ᷴӦ�����ӷ���ʽ ��
��2�����Ӳ����Ǽ����������ƣ�������Һ��pHΪ ��Ŀ���dz�ȥ��Һ��������Al3����Fe3��������Fe(OH)3�Ƿ������ȫ��ʵ�������
��
��3������ʱ���õIJ��������� ��������Ҫ�ɷֵĻ�ѧʽ ��
��4���ữʱ�������Ŀ��Ϊ���� ���ڷ�ֹCa2+ ������ʱ����ˮ�⡣
��5��Ϊʲô�����ᾧҪ������160�棺 ��
��6���ⶨ������Ʒ�Ĵ��ȣ�����һ��Ũ�ȵ�AgNO3��Һ�ζ�һ�������ľ�����Ʒ��������Ʒ��CaCl2 ��2H2O����������ƫ�ߣ���������ʵ���������ܵ�ԭ��֮һΪ ��
��. ��ʵ������������װ�ã����Ʊ�ijЩ���岢��֤�仯ѧ���ʡ�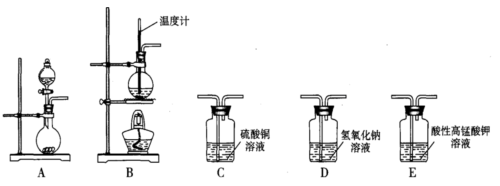
��������
| ��� | ���� | װ������˳������ĸ�� | �Ʊ���Ӧ�Ļ�ѧ����ʽ |
| ��1�� | ��ϩ | _________________ | _________________ |
| ��2�� | ��Ȳ | A��C��E | _________________ |
�������й����ϵ�֪H2S��PH3��������ͭ��Һ��Ӧ�����������ҩƷװ��ͼ���£�
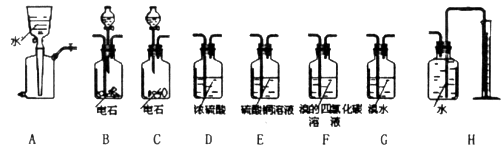
��1������������ҵ�����ѡ����ѵ�װ������ǣ�ѡ����ĸ��ţ���_________
��2��Ϊ��֤�ⶨ�ľ��ȣ�Ҫ�������Ȳ��������Ϊƽ�����ڷ�Һ©����Ӧװ���Һ����________________��
��3������ʯ��Ʒ������Ϊm1g��F������Ȳǰ���������Ϊm2g�����ʯ��Ʒ��CaC2��������
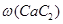 ��_______________��
��_______________�� �±��е�ʵ������ܴﵽʵ��Ŀ�Ļ��ܵó���Ӧ���۵���
| ѡ�� | ʵ������ | ʵ��Ŀ�Ļ�ʵ����� |
| A | ��ʢ��2 mL 0.1 mol/L AgNO3��Һ���Թ��еμ�5��0.1 mol/L NaCl��Һ���а�ɫ�������ɣ��������еμ�5��0.1 mol/L KI��Һ | ˵��һ�ֳ�����ת��Ϊ�ܽ�ȸ�С�ij��� |
| B | ��1 mL 20% ��������Һ�м���3��5��ϡ���ᣬˮԡ����5 min����ȴ���ټ�������Cu(OH)2����Һ������ | ֤�������ܷ���ˮ�ⷴӦ |
| C | ˮԡ����Ũ���ᡢŨ����ͱ��Ļ�����ֱ�������Һ��õ��Ĵֲ�Ʒ | �Ʊ��������� |
| D | ������,�ֱ���2֧�Թ��м�����ͬ�������ͬŨ�ȵ�Na2S2O3��Һ,�ٷֱ����������ͬŨ�ȵ�ϡ���� | �о�Ũ�ȶԷ�Ӧ���ʵ�Ӱ�� |
������ʵ��װ�ý��е�ʵ���У����ܴﵽ��Ӧʵ��Ŀ�ĵ��� (����)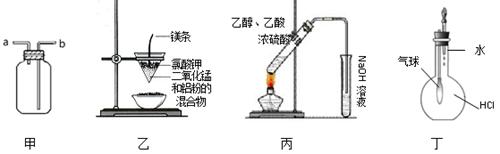
| A��װ�üף�����ӣ�ڽ��룬�ռ�CO2 | B��װ���ң����Ƶý����� |
| C��װ�ñ���ʵ������ȡ�������� | D��װ�ö�����֤HCl������ˮ�е��ܽ��� |
����������ȷ����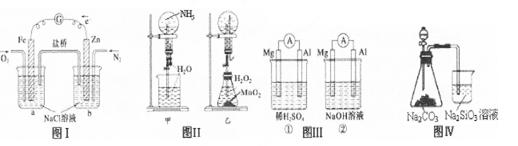
| A��ͼIװ����ͨ��N2��O2����������ͬ�� |
| B��ͼ���мס�����װ�ò�����Ȫ��ԭ��һ�� |
| C��ͼ���װ����þƬ��ԭ��صĸ�������װ����þƬ��ԭ��ص����� |
| D��ͼ����Һ©��ʢ������֤���ǽ�����N��C��Si��ʢ������֤���ǽ�����S��C��Si |
���и���ʵ��������������ó��Ľ�����ȷ����
| ѡ�� | ʵ����� | ʵ������ | ���� |
| A | ȡ�����ʵ��������ֽ�������X��Y���ֱ������������ᷴӦ | X���������������Y�� | �����ԣ�X>Y |
| B | �����pH��3��HA��HB������ֱ���������п��Ӧ����ˮ���ռ����� | HA�ų����������ҷ�Ӧ���ʿ� | HA���Ա�HBǿ |
| C | ��CuSO4��Һ�м���KI��Һ���ټ��뱽���� | �а�ɫ�������ɣ��������ɫ | ��ɫ��������ΪCuI |
| D | ȡ���õ�Na2O2��ĩ�������еμӹ��������� | ������ɫ���� | Na2O2û�б��� |