��Ŀ����
SO2��һ����Ҫ�Ļ���ԭ�ϣ�Ҳ������������Ⱦ����Ҫ��Դ��
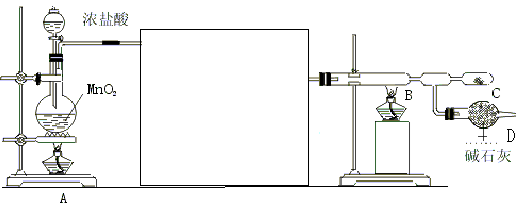
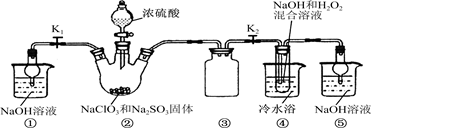
(1)ij��ȤС�������ͼ��ʾװ����ȡSO2
������ʵ�鷽��������ͼ��ʾװ����ȡ����SO2���Լ���_______(����ţ���
| A��Na2SO3��Һ��ϡ���� |
| B��Na2SO3������Ũ���� |
| C������������� |
| D��ͭ��Ũ���� |
��β������װ�õ�����˳����b��( )�� ( )��e��
(2)Ϊ�˻�������SO2�������о���Ա���������õ�Ʒλ���̿�(��Ҫ�ɷ���MnO2)���ո��±��պ������������SO2�������Ʊ������̾���(
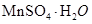 �������̣�������ʾ��ͼ���£�
�������̣�������ʾ��ͼ���£�
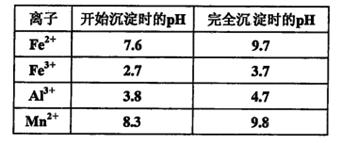
��֪������Һ��pH<2�����еĽ���������Ҫ��Mn2+��������������Fe2����Al3���������������ӡ��йؽ��������γ������������ʱ��Һ��pH���±���
��ش�
�ٺ�Al3�����γ�������ˮ���������ӷ���ʽ��ʾ�侻ˮԭ��________________________��
�ڽ�����������Ҫ��Ӧ�Ļ�ѧ����ʽ��___________________________________________��
�����������м���MnO2�۵�Ŀ����______________________________________________;
��Ӧ�����ӷ���ʽ��_________________________________________________________��
����ʯ�ҽ�����pH��pHӦ���ڵķ�Χ��___________________________________��
����������Ҫ�ɷ���____________________________________��
��1����B (2��)
�ڷ�Һ©�� (2��)
��d c (2��)
��2����Al3++3H2O  Al(OH)3+3H + (2��)
Al(OH)3+3H + (2��)
��SO2��MnO2��MnSO4 (2��)
�۽�Fe2������ΪFe3�� (2��)
2Fe2����MnO2��4H����2Fe3����Mn2����2H2O (2��)
��4.7��pH��8.3 (2��)
��������������������������� ��2�֣�
����

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�ѡ���ʵ�װ�á��Լ��ͷ������Ʊ���ѧ��ѧ�еļ��ֳ������塣����д���еĿոӢ١�����ѡ������ţ���
| ʵ�� | ���� | ����װ�� | �������� | ���������ѡ�õ��Լ� |
| ��1�� | | �� | ��ˮ��Һ�Լ��� | |
| ��2�� | | | 1mol�������2molH2��Ӧ | �� |
��Cl2 ��C2H2
��C2H4 ��NH3
���ʯ�� ��ŨH2SO4
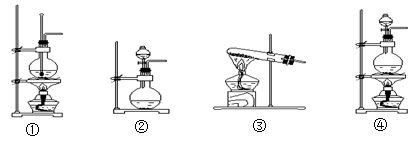
�⻯�ƹ����ǵ�ɽ�˶�Ա���õ���Դ�ṩ����ij��ȤС����ѡ������װ���Ʊ��⻯�ơ�
�ش��������⣺
��1�����װ��E�����ԵIJ��������� ��
��2����������װ����ȡ�⻯��ʱ��������������˳��Ϊi��___��___��___�� �� �� ��a���������ӿڵ���ĸ��ţ���
��3������������ʵ��װ�ý���ʵ�飬ʵ�鲽�����£����װ�������Ժ�װ��ҩƷ����Һ©��������__________________���밴��ȷ��˳���������в���ı�ţ���
| A�����ȷ�Ӧһ��ʱ�� | B���ռ����岢�����䴿�� |
| C���رշ�Һ©������ | D��ֹͣ���ȣ������ȴ |
������ʵ��װ���ܴﵽʵ��Ŀ�ĵ���
| A���Ƚ�NaHCO3��Na2CO3���ȶ��Դ�С | B����ͭ��ϡ������ȡ���ռ�����NO |
| C����֤������ˮ���ܽ�ȵĴ�С | D��ʵ�������Ȼ������������ |

 FeSO4+H2��
FeSO4+H2��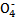 +5C2
+5C2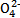 +16H+
+16H+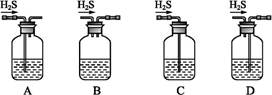


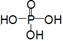 ��__________________________________��
��__________________________________��