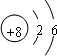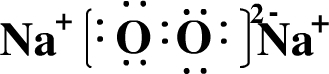��Ŀ����
��A��B��C��D��E���ֶ�����Ԫ�أ�AԪ�ص�һ��ͬλ��ԭ�Ӻ��ڲ������ӣ��䵥��Ϊ���壻Bԭ�������������Ǵ�����������3����C 2+��BԪ�ص����Ӿ�����ͬ�ĵ��Ӳ�ṹ��D��Eԭ�Ӿ�����ͬ�ĵ��Ӳ������Ҵ�����������Ϊ8����ͬ��������Ԫ����ԭ�Ӱ뾶E���D��С���ش��������⣺
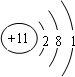
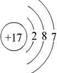
��1��C 2+������Dԭ�ӵĽṹʾ��ͼ�ֱ�Ϊ��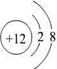
 ��
��
 ��
��
��2��B��E�γɵ����ֻ�����ĵ���ʽ�ǣ�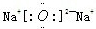
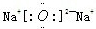 ��
��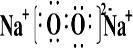
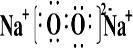
����֮һ����A ��B�γɵĻ����ﷴӦ�õ�����̬B��д���÷�Ӧ�Ļ�ѧ����ʽ��
��3����������Ԫ���еļ����γɵĻ������м�����ǿ����
��1��C 2+������Dԭ�ӵĽṹʾ��ͼ�ֱ�Ϊ��




��2��B��E�γɵ����ֻ�����ĵ���ʽ�ǣ�
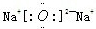
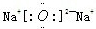
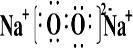
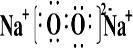
����֮һ����A ��B�γɵĻ����ﷴӦ�õ�����̬B��д���÷�Ӧ�Ļ�ѧ����ʽ��
2Na2O2+2H2O=4NaOH+O2��
2Na2O2+2H2O=4NaOH+O2��
����3����������Ԫ���еļ����γɵĻ������м�����ǿ����
NaOH
NaOH
����ѧʽ����������������ǿ����HClO4
HClO4
����ѧʽ������������A��B��C��D��E���ֶ�����Ԫ�أ�AԪ�ص�һ��ͬλ��ԭ�Ӻ��ڲ������ӣ��䵥��Ϊ���壬��AΪHԪ�أ�Bԭ�������������Ǵ�����������3����������������Ϊ6����BΪOԪ�أ�C 2+��BԪ�ص����Ӿ�����ͬ�ĵ��Ӳ�ṹ����C��������Ϊ8+2+2=12����CΪMgԪ�أ�D��Eԭ�Ӿ�����ͬ�ĵ��Ӳ������Ҵ�����������Ϊ8����D��E���ڵ������ڣ���ͬ��������Ԫ����ԭ�Ӱ뾶E���D��С����EΪNa��DΪCl��Ȼ������Ԫ�ؼ��䵥�ʡ�����������������
����⣺��A��B��C��D��E���ֶ�����Ԫ�أ�AԪ�ص�һ��ͬλ��ԭ�Ӻ��ڲ������ӣ��䵥��Ϊ���壬��AΪHԪ�أ�Bԭ�������������Ǵ�����������3����������������Ϊ6����BΪOԪ�أ�C 2+��BԪ�ص����Ӿ�����ͬ�ĵ��Ӳ�ṹ����C��������Ϊ8+2+2=12����CΪMgԪ�أ�D��Eԭ�Ӿ�����ͬ�ĵ��Ӳ������Ҵ�����������Ϊ8����D��E���ڵ������ڣ���ͬ��������Ԫ����ԭ�Ӱ뾶E���D��С����EΪNa��DΪCl��
��1��þ���ӵĽṹʾ��ͼΪ ����ԭ�ӵĽṹʾ��ͼΪ
����ԭ�ӵĽṹʾ��ͼΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
�� ��
��
��2��B��E�γɵ����ֻ�����ΪNa2O��Na2O2������ʽ�ֱ�Ϊ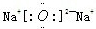 ��
��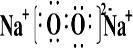 ������������ˮ��Ӧ������������Ӧ�Ļ�ѧ����ʽΪ
������������ˮ��Ӧ������������Ӧ�Ļ�ѧ����ʽΪ
2Na2O2+2H2O=4NaOH+O2����
�ʴ�Ϊ��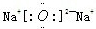 ��
��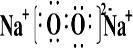 ��2Na2O2+2H2O=4NaOH+O2����
��2Na2O2+2H2O=4NaOH+O2����
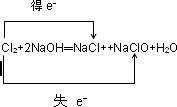
��3������Ԫ���У�Na�Ľ�������ǿ����NaOH�ļ�����ǿ��Cl�ķǽ�������ǿ����HClO4��������ǿ��
�ʴ�Ϊ��NaOH��HClO4��
��1��þ���ӵĽṹʾ��ͼΪ
 ����ԭ�ӵĽṹʾ��ͼΪ
����ԭ�ӵĽṹʾ��ͼΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
�� ��
����2��B��E�γɵ����ֻ�����ΪNa2O��Na2O2������ʽ�ֱ�Ϊ
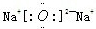 ��
��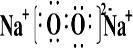 ������������ˮ��Ӧ������������Ӧ�Ļ�ѧ����ʽΪ
������������ˮ��Ӧ������������Ӧ�Ļ�ѧ����ʽΪ2Na2O2+2H2O=4NaOH+O2����
�ʴ�Ϊ��
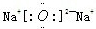 ��
��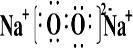 ��2Na2O2+2H2O=4NaOH+O2����
��2Na2O2+2H2O=4NaOH+O2������3������Ԫ���У�Na�Ľ�������ǿ����NaOH�ļ�����ǿ��Cl�ķǽ�������ǿ����HClO4��������ǿ��
�ʴ�Ϊ��NaOH��HClO4��
���������⿼��λ�á��ṹ�����ʵĹ�ϵ��Ӧ�ã���ȷԪ�ص��ƶ��ǽ����Ĺؼ�����ע��Ԫ�ص����ʼ���ѧ�������ȷʹ������ɣ��Ѷ��еȣ�

��ϰ��ϵ�д�
 ͬ����ϰ���ϴ�ѧ������ϵ�д�
ͬ����ϰ���ϴ�ѧ������ϵ�д� ͬ����ϰ����ʦ����ѧ������ϵ�д�
ͬ����ϰ����ʦ����ѧ������ϵ�д� ����ϰ�⽭��ϵ�д�
����ϰ�⽭��ϵ�д� ѧ���쳵��������������������ϵ�д�
ѧ���쳵��������������������ϵ�д�
�����Ŀ