��Ŀ����
��NA��ʾ�����ӵ�������ֵ������������ȷ����
| A���ڷ�ӦKIO3 + 6 HI��KI + 3I2��3 H2O�У�ÿ����3mo1 I2ת�Ƶĵ�����Ϊ5NA |
| B��100mL 18.4mo1��L-1����������Cu��Ӧ������SO2�ķ�����Ϊ0.92NA |
| C��1L 0.1 moI��L-1��CH3COOH��Һ�����������Ӻͷ�������Ϊ0.1NA |
| D����0.lmol FeC13���˷�ˮ�п��Ƶ�0.1NA Fe(OH)3���� |
A
���������A���ڷ�ӦKIO3 + 6 HI��KI + 3I2��3 H2O�У��������ǵ���أ���Ԫ�صĻ��ϼ۴���5�۽��͵�0�ۣ��õ�5�� ���ӣ�����ÿ����3mo1 I2ת�Ƶĵ�����Ϊ5NA��A��ȷ��B����ͭ��Ũ����ķ�Ӧ�У����ŷ�Ӧ�Ľ��У������Ũ�����ͣ�ϡ������ͭ����Ӧ�����100mL 18.4mo1��L-1����������Cu��Ӧ������SO2�ķ�����С��0.92NA��B����ȷ��C��������Һ�к��д�����ˮ���ӣ����1L 0.1 moI��L-1��CH3COOH��Һ�����������Ӻͷ�����������0.1NA��C����ȷ��D��������ˮ���ǿ��淴Ӧ�����Խ�0.lmol FeC13���˷�ˮ���Ƶõ�Fe(OH)3������С��0.1NA��D����ȷ����ѡA��

��ϰ��ϵ�д�
�����Ŀ
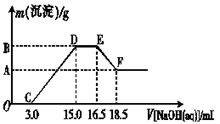
 ���������������Ҫ��ԴΪȼú��������β���ȡ�ijʵ��С�齫PM2��5����������ˮ�����Ƴɴ�����������ø����������ԣ��������Ũ�����±������ݱ������ݿ�֪����ҺPHΪ
���������������Ҫ��ԴΪȼú��������β���ȡ�ijʵ��С�齫PM2��5����������ˮ�����Ƴɴ�����������ø����������ԣ��������Ũ�����±������ݱ������ݿ�֪����ҺPHΪ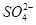
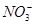
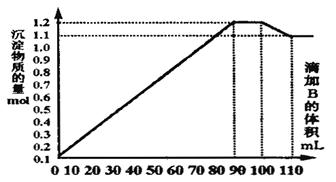
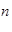 A��ʾ�����ӵ�������ֵ������˵����ȷ����
A��ʾ�����ӵ�������ֵ������˵����ȷ����