题目内容
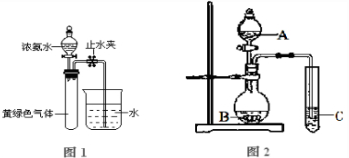
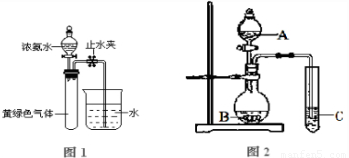
已知 NH3和 Cl2在常温下可快速反应生成氮气:2NH3+3Cl2→N2+6HCl.当 Cl2和 NH3 比例不同时,产物有差异.
(1)若利用该反应处理含有氨气和氯气的尾气,用于制备盐酸,则 Cl2和 NH3的最佳比例为 .该反应可用于检验化工生产中氯气是否泄漏.如氯气有少量泄漏,用氨气检验时有明显现象,此过程中发生反应的Cl2和NH3的体积比范围为 .
(2)体积为1.12L,质量为3.335g的Cl2和N2的混合气体通过浓氨水后,体积变为0.672L(其中Cl2体积分数为50%)(气体体积均为标准状况下测定).则原混合气体中N2的体积为 mL.
(3)根据第(2)题数据,计算被氧化的NH3的质量.下面是两位学生从不同角度解题时所列的第一步算式,请判断他们所列未知数X分别表示什么量,并填写在表格内:(单位没列出)
(4)有Cl2和N2的混合气体,其中N2的体积分数为x,将1L该混合气体与1L氨气混合,讨论x取不同范围的数值时,所得气体体积y与x的关系式(所有气体体积均在相同状况下测定).
(1)若利用该反应处理含有氨气和氯气的尾气,用于制备盐酸,则 Cl2和 NH3的最佳比例为
(2)体积为1.12L,质量为3.335g的Cl2和N2的混合气体通过浓氨水后,体积变为0.672L(其中Cl2体积分数为50%)(气体体积均为标准状况下测定).则原混合气体中N2的体积为
(3)根据第(2)题数据,计算被氧化的NH3的质量.下面是两位学生从不同角度解题时所列的第一步算式,请判断他们所列未知数X分别表示什么量,并填写在表格内:(单位没列出)
| 学生编号 | 所列第一步算式 | 未知数X表示的意义 | ||||
| 甲 |
|
|||||
| 乙 |
|
分析:(1)NH3和 Cl2在常温下可快速反应生成氮气:2NH3+3Cl2→N2+6HCl,利用该反应处理含有氨气和氯气的尾气,Cl2和 NH3的最佳比例是恰好完全反应;检验氯气存在需要氯气和氨气反应生成氯化氢和氨气冒白烟生成氯化铵白色固体,氨气过量;
(2)标准状况下体积为1.12L,质量为3.335g的Cl2和N2的混合气体的平均摩尔质量,结合质量和物质的量计算得到原混合气体中的氯气和氮气物质的量之比,计算得到氮气体积;
(3)依据图表中的计算式和依据分析判断;
(4)依据反应的极值方法进行分析计算分析判断不同范围的气体体积,注意反应前后差量的计算应用.
(2)标准状况下体积为1.12L,质量为3.335g的Cl2和N2的混合气体的平均摩尔质量,结合质量和物质的量计算得到原混合气体中的氯气和氮气物质的量之比,计算得到氮气体积;
(3)依据图表中的计算式和依据分析判断;
(4)依据反应的极值方法进行分析计算分析判断不同范围的气体体积,注意反应前后差量的计算应用.
解答:解:(1)氨气和氯气在常温下可快速反应生成氮气:2NH3+3Cl2=N2+6HCl.若利用该反应处理含有氨气和氯气的尾气,用于制备盐酸,则 Cl2和 NH3的最佳比例为3:2;如果管道漏气,则发生反应3Cl2+2NH3=N2+6HCl,生成HCl气体继续与氨气反应生成氯化铵,现象为有白烟生成,即氨气应过量,所以发生反应的Cl2和NH3的体积比为大于0,小于1.5,故答案为:3:2; 大于0,小于1.5;
(2)体积为1.12L物质的量为0.05mol,质量为3.335g的Cl2和N2的混合气体的平均摩尔质量=
=66.7g/mol,设混合气体中含有氮气物质的量为X,氯气物质的量为(0.05-X)mol,得到28X+(0.05-X)71=3.335,计算得到X=0.005mol,则原混合气体中N2的体积为0.005mol×22,4L/mol=0.112L=112ml;
故答案为:112;
(3)甲:2NH3+3Cl2→N2+6HCl计算表达式是利用反应前后气体物质的量的变化进行计算,所以计算式中X的意义是被氧化的氨气的物质的量;
故答案为:被氧化氨的物质的量;
乙:2NH3+3Cl2→N2+6HCl分析反应和计算表达式分析判断,X为原混合气体中氯气的体积,故答案为:反应前Cl2的体积;
(4)若按上式反应,2NH3+3Cl2→N2+6HCl,氨过量,所以还会生成氯化铵8NH3+3Cl2→N2 +6NH4Cl;当x=62.5%时恰好生成氯化铵和氮气,此时Y=0.75L(生成0.125+原来的0.625.也可用差量法2-1.25);当x>62.5%时剩余氨气,可以用氯气计算,差量法求得气体减少10(1-x)/3,所以Y=2-10(1-x)/3=(10x-4)/3;当x<62.5%时氯和氨都用完,求出生成的氮气和氯化氢气体体积之和为(2-3x),原有氮x,所以共有气体体积为Y=(2-2x)
答:关系式为y=2-2x 0.625>x>0;y=(10x-4)/3 x≥0.625.
(2)体积为1.12L物质的量为0.05mol,质量为3.335g的Cl2和N2的混合气体的平均摩尔质量=
| 3.335g |
| 0.05mol |
故答案为:112;
(3)甲:2NH3+3Cl2→N2+6HCl计算表达式是利用反应前后气体物质的量的变化进行计算,所以计算式中X的意义是被氧化的氨气的物质的量;
故答案为:被氧化氨的物质的量;
乙:2NH3+3Cl2→N2+6HCl分析反应和计算表达式分析判断,X为原混合气体中氯气的体积,故答案为:反应前Cl2的体积;
(4)若按上式反应,2NH3+3Cl2→N2+6HCl,氨过量,所以还会生成氯化铵8NH3+3Cl2→N2 +6NH4Cl;当x=62.5%时恰好生成氯化铵和氮气,此时Y=0.75L(生成0.125+原来的0.625.也可用差量法2-1.25);当x>62.5%时剩余氨气,可以用氯气计算,差量法求得气体减少10(1-x)/3,所以Y=2-10(1-x)/3=(10x-4)/3;当x<62.5%时氯和氨都用完,求出生成的氮气和氯化氢气体体积之和为(2-3x),原有氮x,所以共有气体体积为Y=(2-2x)
答:关系式为y=2-2x 0.625>x>0;y=(10x-4)/3 x≥0.625.
点评:本题考查了氯气性质的分析判断,化学方程式的差量计算应用,计算式的分析判断未知量的含义,题目难度较大.

练习册系列答案
相关题目