��Ŀ����
�������ȣ�ClO2����Ϊһ�ָ�Чǿ�������ѱ����Ϲ�����������֯��WHO����ΪAI����ȫ�������������¶�������Ϊ����ɫ���ٻ�ɫ���壬���ʷdz����ȶ����¶ȹ���ˮ��Һ��ClO2��������������30���Ⱦ��п�������ը�������Һ��Ӧ�����κ�ˮ��

��1��ij�о�С�������ͼ��ʾʵ���Ʊ�ClO2��Һ���䷴Ӧ�Ļ�ѧ����ʽΪ
![]()
���ڷ�Ӧ��ʼ֮ǰ���ձ��е�ˮ���ȵ�80�棬Ȼ��ֹͣ���ȣ���ʹ���¶ȱ�����60��80��֮�䡣�����¶ȵ�Ŀ���� ��ͼʾװ����ȱ�ٵ�һ�ֱ���IJ��������� ��
��װ��A�����ܽ�����Ķ����������壬�������ʢ�� ������ĸ����
A��20mL 60�����ˮ B��100mL��ˮ
C��100mL����ʳ��ˮ D��100mL��ˮ
������ƿ�м���12��25g KClO3��9g���ᣨH2C2O4����Ȼ���ټ���������ϡ���ᣬˮԡ���ȣ���Ӧ������ClO2������Ϊ ��
��2����ClO2������������ˮ��pHΪ5��5��6��5��������һ���������岻��������������ӣ�![]() ��������ˮ��ClO2��
��������ˮ��ClO2��![]() �ĺ��������������������вⶨ��ʵ�鲽�����£�
�ĺ��������������������вⶨ��ʵ�鲽�����£�
����1��ȷ��ȡһ�������ˮ��������ƿ�У�
����2������ˮ����pH��7��0��8��0��
����3������������KI���壻
����4����������ָʾ������һ��Ũ�ȵ�Na2S2O3��Һ�ζ����յ㣻
����5���ٵ�����Һ��pH��2��0��
����6����������ͬŨ�ȵ�Na2S2O3��Һ�ζ����յ㡣
�ٲ���1����Ҫ��ȡ20��00mLˮ������Ӧѡ�õ�������
�ڲ���1��4��Ŀ���Dzⶨˮ����ClO2�ĺ������䷴Ӧ�Ļ�ѧ����ʽΪ:
![]() ����4�м����ָʾ��Ϊ ���ζ��ﵽ�յ�ʱ��Һ����ɫ�仯Ϊ
����4�м����ָʾ��Ϊ ���ζ��ﵽ�յ�ʱ��Һ����ɫ�仯Ϊ
�۲���5��Ŀ����ʹ![]() ����Һ�е�
����Һ�е�![]() ��ԭΪ
��ԭΪ![]() �Բⶨ�京�����÷�Ӧ�����ӷ���ʽΪ�� ��
�Բⶨ�京�����÷�Ӧ�����ӷ���ʽΪ�� ��
��������ˮ��![]() �ĺ������꣬�������м���������
�ĺ������꣬�������м���������![]() ��
��![]() ��ԭΪ
��ԭΪ![]() ����÷�Ӧ����������Ϊ ���ѧʽ��
����÷�Ӧ����������Ϊ ���ѧʽ��
��1����ʹ��Ӧ�������У�����ֹ�¶ȹ�������ը��2�֣����¶ȼƣ�1�֣�
��b��2�֣� ��6.75g��2�֣�

 һ����ʦȨ����ҵ��ϵ�д�
һ����ʦȨ����ҵ��ϵ�д� �������ȣ�ClO2��Ϊһ�ֻ���ɫ���壬�ǹ����Ϲ��ϵĸ�Ч�����ס����١���ȫ��ɱ����������
�������ȣ�ClO2��Ϊһ�ֻ���ɫ���壬�ǹ����Ϲ��ϵĸ�Ч�����ס����١���ȫ��ɱ����������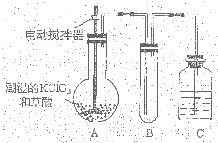 �������ȣ�ClO2����һ�ֻ���ɫ�д̼�����ζ�����壬���۵�Ϊ-59�棬�е�Ϊ11.0�棬������ˮ��ClO2���Կ����������ᣨHClO2�������ᣨHClO3���Ļ����������ҵ�����Գ�ʪ��KClO3�Ͳ��ᣨH2C2O4����60��ʱ��Ӧ�Ƶã�ijѧ��������ͼ��ʾװ��ģ�ҵ��ȡ���ռ�ClO2�����г�������ʡ�ԣ����ش����⣺
�������ȣ�ClO2����һ�ֻ���ɫ�д̼�����ζ�����壬���۵�Ϊ-59�棬�е�Ϊ11.0�棬������ˮ��ClO2���Կ����������ᣨHClO2�������ᣨHClO3���Ļ����������ҵ�����Գ�ʪ��KClO3�Ͳ��ᣨH2C2O4����60��ʱ��Ӧ�Ƶã�ijѧ��������ͼ��ʾװ��ģ�ҵ��ȡ���ռ�ClO2�����г�������ʡ�ԣ����ش����⣺