��Ŀ����
12���¶�ΪT�棬ѹǿΪ1.01��106Pa�����£�ij�ܱ��������з�Ӧ�ﵽ��ѧƽ��A��g��+aB������?4C��g�����ﵽ��ѧƽ��ʱ���c��A��=0.2mol•L-1��ѹ������ʹѹǿ����2.02��106Pa���ڶ��δﵽƽ��ʱ�����c��A��=0.36mol•L-1��������ѹ��������ʹѹǿ����5.05��106Pa�������δﵽƽ��ʱ�����c��A��=1.1mol•L-1�������й����й�˵������ȷ���ǣ�������| A�� | �ڶ���ƽ��ʱBΪ��̬ | |
| B�� | a��3 | |
| C�� | ��һ��ƽ�������ѹǿƽ�������ƶ� | |
| D�� | �����δﵽƽ��ʱB����Ϊ����̬ |
���� AһֱΪ��̬����ʼc��A��=0.2mol/L��ѹ������ʹѹǿ����2.02��106Pa���൱�������Сһ�룬��ƽ���ƶ�֮ǰ��c��A��=0.4mol/L��ͬ��������ƽ��ǰ�������СΪ��һ�ε�$\frac{1}{5}$������c��A����ƽ���ƶ�֮ǰ��Ũ��Ӧ��0.2x5=1mol/L�����ѹǿ��ƽ���ƶ���Ӱ���Լ�ʵ��A��Ũ�����������
��� �⣺AһֱΪ��̬����ʼc��A��=0.2mol/L��ѹ������ʹѹǿ����2.02��106Pa���൱�������Сһ�룬��ƽ���ƶ�֮ǰ��c��A��=0.4mol/L���ڶ��δﵽƽ��ʱ��
���c��A��=0.36mol/L˵��ƽ�������ƶ���ѹǿ���ӣ�ƽ����������ķ����ƶ���˵��B�����壬a��3��ͬ��������ƽ��ǰ�������СΪ��һ�ε�$\frac{1}{5}$������c��A����ƽ���ƶ�֮ǰ��Ũ��Ӧ��0.2x5=1mol/L������ƽ��ʱc��A��Ϊ1.1mol/L����ƽ���������ƶ�����ʱB�Ƿ����壮
��ѡC��
���� ������һ������Ӱ�컯ѧƽ���ƶ������ص�֪ʶ�Ŀ����⣬����ѧ�������ͽ��������������Ѷ��еȣ�

��ϰ��ϵ�д�
 ���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д�
���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д� ����ѵ��ϵ�д�
����ѵ��ϵ�д� ��ĩ�����ϵ�д�
��ĩ�����ϵ�д�
�����Ŀ
2��������Ϊ27��Ԫ��R���������14�����ӣ���R���γɵ�����Ϊ��������
| A�� | R+ | B�� | R2+ | C�� | R3+ | D�� | R2- |
3��2013��4�£�����˹������5����̽�¼ƻ�������������ʱ���Իͣ������������������������ֵ�${\;}_{\;}^{3}$He��ÿ�ٶ�${\;}_{\;}^{3}$He�˾۱����ͷų��������൱��Ŀǰ����һ�����ĵ������� ����˵������ȷ���ǣ�������
| A�� | ${\;}_{\;}^{3}$Heԭ���е�������ԭ�Ӻ���ռ���һ��������ƺ˸����˶� | |
| B�� | ${\;}_{\;}^{3}$Heԭ�Ӳ���С��ʵ������ | |
| C�� | ${\;}_{\;}^{3}$Heԭ���Dz����ٷֵ��� | |
| D�� | ${\;}_{\;}^{3}$He������������Ϊ2����${\;}_{\;}^{3}$He���н�ǿ�Ľ����� |
7������˵������ȷ���ǣ�������
| A�� | ��50mL��Ͳ������0.1000mo1•L-1̼������Һ | |
| B�� | 0.5mol O3��11.2L O3�����ķ�����һ����� | |
| C�� | ��������ΪNA��NO2��CO2��������к��е���ԭ����Ϊ2NA | |
| D�� | ���³�ѹ�£�22.4L��NO2��CO2������庬��2NA����ԭ�� |
3��ʵ�������ñ���ȩ�Ʊ����״��ͱ����ᣮ
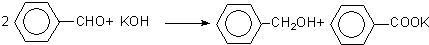
��֪�����Ʊ�ԭ��Ϊ��
���й����ʵ����������
��ȩ�����뱥��NaHSO3��Һ��Ӧ�����������л����������ˮ�����Σ�
�ܴ�����Һ̬�л���һ�㶼�й̶��е㣮
�Ʊ����״��ͱ��������Ҫ������ͼ��

��1������I�������Ƿ�Һ������II������������
��2������III�������ǹ��ˣ���Ʒ���DZ����ᣮ
��3��ijͬѧ�ⶨ��Ʒ�ķе㣬��������190�漴��ʼ���ڣ���ͬѧ�Ʋ��Ʒ���DZ��״��뱽��ȩ�Ļ�����������·������м�����ᴿ����������Ʋ���ȷ�����ڴ������ɱ������ݣ�
��4�����Ȳⶨ����ȡ7.00g��Ʒ�ף���������������Һ��ַ�Ӧ�����ɵ���Ag 2.16g�����Ʒ���б��״������������ļ������ʽΪ��$\frac{7.00g-\frac{2.16g}{108g/mol}��\frac{1}{2}��106g/mol}{7.00g}$100%��
������Ϊ84.9%������������Ч���֣�����ȩ��Է���������106��Ag��108����
��֪�����Ʊ�ԭ��Ϊ��

���й����ʵ����������
| ���� | �е� | �۵� | �ܽ��� |
| ����ȩ | 179�� | -26�� | ����ˮ���������ѻ��ܣ� |
| ���״� | 205.3�� | -15.3�� | ������ˮ���������ѻ��� |
| ������ | 249�� | 122�� | ����ˮ�����������ѣ� |
| ���� | 34.8�� | ������ˮ |
�ܴ�����Һ̬�л���һ�㶼�й̶��е㣮
�Ʊ����״��ͱ��������Ҫ������ͼ��

��1������I�������Ƿ�Һ������II������������
��2������III�������ǹ��ˣ���Ʒ���DZ����ᣮ
��3��ijͬѧ�ⶨ��Ʒ�ķе㣬��������190�漴��ʼ���ڣ���ͬѧ�Ʋ��Ʒ���DZ��״��뱽��ȩ�Ļ�����������·������м�����ᴿ����������Ʋ���ȷ�����ڴ������ɱ������ݣ�
| ��� | ʵ�鷽�� | ʵ������ | ���� |
| ����� | ȡ������Ʒ���ڽྻ���Թ��У��ټ���2mLAg��NH3��OH ��Һ��ˮԡ���ȣ� | �Թ��ڱڲ����������� | ��Ʒ���к��б���ȩ |
| ����� | ��������Ʒ�����ڷ�Һ©���У�Ȼ����©���м��뱥��NaHSO3��Һ����Һ����ַ�Ӧ���Һ�� | ||
| ����� | ������ڵ��л���ϴ�ӡ�����ⶨ��Ʒ�� �е� | �е�Ϊ205.3�� | �����л����Ǵ����ı��״� |
������Ϊ84.9%������������Ч���֣�����ȩ��Է���������106��Ag��108����
10�����и����л��������ˮ��Һ������������������ͭ����Һ������ǣ�������
| A�� | �����Ǻ���ȩ | B�� | ��ȩ�ͱ�ͪ | C�� | ��ȩ������ | D�� | ���Ǻ������� |
8��������Ԫ���У�����������Ԫ�ص��ǣ�������
| A�� | �� | B�� | �� | C�� | �� | D�� | �� |