��Ŀ����
��13�֣�A��B��C��D��E��F���ֻ��������A��B��C��D��E���ɶ�����Ԫ����ɣ���ɫ��Ӧ��Ϊ��ɫ��B��C��E��������Ԫ����ɡ�B��C�����Ԫ����ͬ����C��Ħ��������B��80g/mol ���ش�
��1�����廯����AΪdz��ɫ��ĩ���û������к��еĻ�ѧ��Ϊ
A�����Ӽ� B�����Թ��ۼ� C���Ǽ��Թ��ۼ� D�����
���� �±�ΪB��Fʵ��IJ�������
д��B��ϡ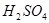 ��Ӧ�����ӷ���ʽ
��Ӧ�����ӷ���ʽ
д�����з�Ӧ�Ļ�ѧ����ʽ
��3������6������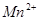 ��
�� ��
�� ��
�� ��
��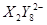 ��C�к��е������ӣ���
��C�к��е������ӣ���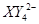 ���һ�����ӷ���ʽ����֪
���һ�����ӷ���ʽ����֪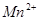 Ϊ��ԭ�����õ�1mol
Ϊ��ԭ�����õ�1mol �������������ʵ���Ϊ mol
�������������ʵ���Ϊ mol
��4��������D��E�ת��D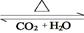 E������D��E ��
E������D��E ��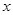 H2O�Ļ����13.04g�����ȵ���ȫ��Ӧ���������ͨ��ŨH2SO4����3.42g��ʣ������ͨ����ʯ������2.20g����������D������Ϊ g��E ��
H2O�Ļ����13.04g�����ȵ���ȫ��Ӧ���������ͨ��ŨH2SO4����3.42g��ʣ������ͨ����ʯ������2.20g����������D������Ϊ g��E ��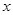
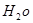 �Ļ�ѧʽΪ
�Ļ�ѧʽΪ
��1�����廯����AΪdz��ɫ��ĩ���û������к��еĻ�ѧ��Ϊ
A�����Ӽ� B�����Թ��ۼ� C���Ǽ��Թ��ۼ� D�����
���� �±�ΪB��Fʵ��IJ�������
���ں�B����Һ�м���ϡ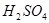 ������dz��ɫ���Ǻ�ʹ����ʯ��ˮ����ǵ���ɫ�д̼�����ζ������ ������dz��ɫ���Ǻ�ʹ����ʯ��ˮ����ǵ���ɫ�д̼�����ζ������ |
| ��20mL��ˮ�еμ�F�ı�����Һ1~2mL����Һ��ʺ��ɫ |
| �۽�ʵ��ڵõ��ĺ��ɫҺ��������������գ����յõ�����ɫ���� |
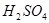 ��Ӧ�����ӷ���ʽ
��Ӧ�����ӷ���ʽ д�����з�Ӧ�Ļ�ѧ����ʽ
��3������6������
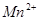 ��
�� ��
�� ��
�� ��
��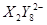 ��C�к��е������ӣ���
��C�к��е������ӣ���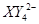 ���һ�����ӷ���ʽ����֪
���һ�����ӷ���ʽ����֪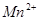 Ϊ��ԭ�����õ�1mol
Ϊ��ԭ�����õ�1mol �������������ʵ���Ϊ mol
�������������ʵ���Ϊ mol��4��������D��E�ת��D
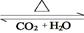 E������D��E ��
E������D��E ��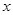 H2O�Ļ����13.04g�����ȵ���ȫ��Ӧ���������ͨ��ŨH2SO4����3.42g��ʣ������ͨ����ʯ������2.20g����������D������Ϊ g��E ��
H2O�Ļ����13.04g�����ȵ���ȫ��Ӧ���������ͨ��ŨH2SO4����3.42g��ʣ������ͨ����ʯ������2.20g����������D������Ϊ g��E ��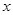
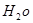 �Ļ�ѧʽΪ
�Ļ�ѧʽΪ ��1��AC(2��)��2��S2O32- + 2H+ =" S��" + SO2 + H2O(2��)
Fe3+ + 3H2O Fe(OH)3(����)+3H+(2��)
Fe(OH)3(����)+3H+(2��)
��3��2.5(2��) ��4��8.4 (2��) Na2CO3��7H2O(3��)
Fe3+ + 3H2O
 Fe(OH)3(����)+3H+(2��)
Fe(OH)3(����)+3H+(2��)��3��2.5(2��) ��4��8.4 (2��) Na2CO3��7H2O(3��)
��1��������ɫ��֪A�ǹ������ƣ����еĻ�ѧ�������Ӽ��ͷǼ��Լ���
��2������B��ϡ���ᷴӦ�������֪��B��Na2S2O3����ӦʽΪS2O32- + 2H+ =" S��" + SO2 + H2O�����ݷ�Ӧ�ڢۿ�֪F���Ȼ�������ӦʽΪFe3+ + 3H2O Fe(OH)3(����)+3H+��
Fe(OH)3(����)+3H+��
��3��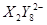 ΪS2O82������ԭ������SO42����S�Ļ��ϼ�����1����λ��1mol��ԭ��ʧȥ5mol���ӣ����Ը��ݵ�ʧ�����غ��֪����Ҫ�����������ʵ�����2.5mol��
ΪS2O82������ԭ������SO42����S�Ļ��ϼ�����1����λ��1mol��ԭ��ʧȥ5mol���ӣ����Ը��ݵ�ʧ�����غ��֪����Ҫ�����������ʵ�����2.5mol��
��4��D��̼�����ƣ��ֽ�����CO2��H2O�Լ�̼���ơ��������ͨ��ŨH2SO4����3.42g����������ˮ��3.420g��ʣ������ͨ����ʯ������2.20g����CO2��2.20g�����ʵ�����0.05mol����̼��������0.1mol��������8.4g���ֽ����ɵ�ˮ��0.05mol��������0.9g��E ��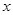 H2O��ˮ��������2.52g�����ʵ�����0.14mol��Na2CO3��XH2O��������13.04g��8.4g��4.64g������̼���Ƶ�������4.64g��2.52g��2.12g�����ʵ�����0.02mol��������֮����1�U7�����Ի�ѧʽΪNa2CO3��7H2O��
H2O��ˮ��������2.52g�����ʵ�����0.14mol��Na2CO3��XH2O��������13.04g��8.4g��4.64g������̼���Ƶ�������4.64g��2.52g��2.12g�����ʵ�����0.02mol��������֮����1�U7�����Ի�ѧʽΪNa2CO3��7H2O��
��2������B��ϡ���ᷴӦ�������֪��B��Na2S2O3����ӦʽΪS2O32- + 2H+ =" S��" + SO2 + H2O�����ݷ�Ӧ�ڢۿ�֪F���Ȼ�������ӦʽΪFe3+ + 3H2O
 Fe(OH)3(����)+3H+��
Fe(OH)3(����)+3H+����3��
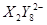 ΪS2O82������ԭ������SO42����S�Ļ��ϼ�����1����λ��1mol��ԭ��ʧȥ5mol���ӣ����Ը��ݵ�ʧ�����غ��֪����Ҫ�����������ʵ�����2.5mol��
ΪS2O82������ԭ������SO42����S�Ļ��ϼ�����1����λ��1mol��ԭ��ʧȥ5mol���ӣ����Ը��ݵ�ʧ�����غ��֪����Ҫ�����������ʵ�����2.5mol����4��D��̼�����ƣ��ֽ�����CO2��H2O�Լ�̼���ơ��������ͨ��ŨH2SO4����3.42g����������ˮ��3.420g��ʣ������ͨ����ʯ������2.20g����CO2��2.20g�����ʵ�����0.05mol����̼��������0.1mol��������8.4g���ֽ����ɵ�ˮ��0.05mol��������0.9g��E ��
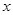 H2O��ˮ��������2.52g�����ʵ�����0.14mol��Na2CO3��XH2O��������13.04g��8.4g��4.64g������̼���Ƶ�������4.64g��2.52g��2.12g�����ʵ�����0.02mol��������֮����1�U7�����Ի�ѧʽΪNa2CO3��7H2O��
H2O��ˮ��������2.52g�����ʵ�����0.14mol��Na2CO3��XH2O��������13.04g��8.4g��4.64g������̼���Ƶ�������4.64g��2.52g��2.12g�����ʵ�����0.02mol��������֮����1�U7�����Ի�ѧʽΪNa2CO3��7H2O��
��ϰ��ϵ�д�
 ͬ��������ϰϵ�д�
ͬ��������ϰϵ�д� �ο�ͨ�γ̱�˼ά����������ѵ��ϵ�д�
�ο�ͨ�γ̱�˼ά����������ѵ��ϵ�д�
�����Ŀ