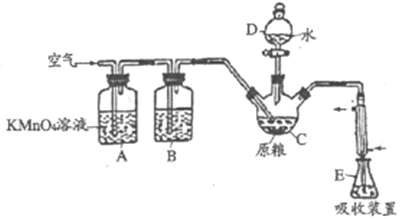
��Ŀ����
����Ŀ��������Ҫ�ĵ��ʣ��ϳ�ԭ��Ϊ�� N2(g)+3H2(g)![]() 2NH3(g) ��H= ��92.4 kJ/mol����500�桢20 MPaʱ����N2��H2����һ���ݻ�Ϊ2 L���ܱ������з�����Ӧ����Ӧ�����и������ʵ����仯��ͼ���ش��������⣺
2NH3(g) ��H= ��92.4 kJ/mol����500�桢20 MPaʱ����N2��H2����һ���ݻ�Ϊ2 L���ܱ������з�����Ӧ����Ӧ�����и������ʵ����仯��ͼ���ش��������⣺
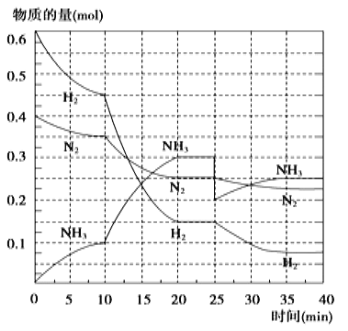
��1��10 min����NH3��ʾ��ƽ����Ӧ���ʣ�______��
��2����10 ~20 min�ڣ�NH3Ũ�ȱ仯��ԭ�������______________
A�����˴��� B����С������� C�������¶� D������NH3���ʵ���
��3����1��ƽ���ƽ�ⳣ��K1 = _________________�������ݵı���ʽ������2��ƽ��ʱNH3���������=___________��С�������һλ����
��4���ڷ�Ӧ������25 minʱ�������߷����仯��ԭ��______________���� ��ڶ���ƽ��ʱ����ƽ���ƽ�ⳣ��K2 ____ K1������������������������С��������
���𰸡�0.005 mol/(L��min) A ![]() 45.5 % ����0.1 mol NH3 ����
45.5 % ����0.1 mol NH3 ����
��������
(1)���ݷ�Ӧ����= ![]() ���㣻
���㣻
(2)����ͼ��֪����Ӧ���ʼӿ죬��10minʱ����������ʵ����������ģ�������������ı仯���ж�ƽ��ʩ�ʷ����ƶ����ٽ��Ӱ��ƽ������ط������
(3)��ѧƽ�ⳣ������������Ũ����֮���뷴Ӧ��Ũ����֮���ıȣ���2��ƽ��ʱ������NH3������������ڰ��������ʵ����������㣻
(4)25���ӣ�NH3�����ʵ���ͻȻ���٣���H2��N2�����ʵ������䣬˵��Ӧ�Ƿ����NH3������ƽ�ⳣ����Ӱ�����ط����жϡ�
(1)��Ӧ����v(NH3)= ![]() =
= ![]() =0.005mol/(L��min)���ʴ�Ϊ��0.005mol/(L��min)��
=0.005mol/(L��min)���ʴ�Ϊ��0.005mol/(L��min)��
(2)��ͼ���֪����������ʵ����仯���ӣ�����Ӧ���ʼӿ죬��10minʱ�仯�������ģ�20min��ƽ��ʱ����n(N2)=0.35mol-0.25mol =0.1mol����n(H2)=0.45mol-0.15mol=0.3mol����n(NH3)=0.3mol-0.1mol =0.2mol�����ʵ����仯֮�ȵ��ڻ�ѧ������֮�ȣ������������ʵ��������ӱ�����ͬ��˵��10min���ܸı��������ʹ�ô�������С����൱������ѹǿ��Ӧ�÷�Ӧ����������ӱ������������¶ȣ�Ӧ�÷�Ӧ���ʼ�С������NH3���ʵ������淴Ӧ�������ӵı���������ֻ��ʹ�ô������ϣ���ѡA��
(3)��ͼ����Կ���������Ӧ���е�ʱ20-25min�������ʵ������䣬˵����Ӧ�ﵽƽ��״̬����ѧƽ�ⳣ������������Ũ����֮���뷴Ӧ��Ũ����֮���ıȣ���ͼ���֪��20min��ƽ��ʱ��n(N2)=0.025mol��10=0.25mol��n(H2)=0.025mol��6=0.15mol��n(NH3)=0.025mol��12=0.3mol������������ƽ�ⳣ��K= ![]() =
=  =
=![]() ��35minʱ�ﵽ��2��ƽ����NH3���������=
��35minʱ�ﵽ��2��ƽ����NH3���������= ![]() ��100%=45.5%���ʴ�Ϊ��
��100%=45.5%���ʴ�Ϊ��![]() ��45.5%��
��45.5%��
(4)��25���ӣ�NH3�����ʵ���ͻȻ���٣���H2��N2�����ʵ������䣬˵��Ӧ�Ƿ����NH3����ͼ����Կ���������Ӧ���е�ʱ35-40min�������ʵ������䣬˵����Ӧ�ﵽ�ڶ���ƽ��״̬��ƽ�ⳣ��ֻ���¶�Ӱ�죬�¶Ȳ��䣬ƽ�ⳣ�����䣬���Գ�ȥ0.1mol������ʱƽ�ⳣ��K�����䣬�ʴ�Ϊ�������0.1molNH3�����ڡ�