��Ŀ����
���ͼ1��ѡ������װ�ã����ŨH2SO4��ľ̿�ڼ���ʱ�ķ�Ӧ�����鷴Ӧ������Ӻ̶������õIJ����ܡ����ܡ����С�����̨������װ�õȾ�ʡ�ԣ���
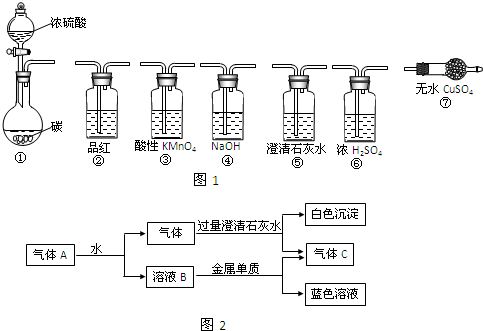
��1��ŨH2SO4��ľ̿�ڼ���ʱ������Ӧ�Ļ�ѧ����ʽ�ǣ�
��2��ѡ�õ�ʵ��װ�ú�����˳����
��3��֤����CO2���ɵ�ʵ��������
��4��װ�â�Ҳ��������Ũ������ľ̿����ʱ������Ӧ����Ӧ�������������A����ͼ2��ʾ�ı仯���ش��������⣺
������C��
��B��ϡ��Һ��������ʷ�Ӧ������ɫ��Һ�����ӷ���ʽ�ǣ�

�������ת�Ƶķ����������B�ڷ�Ӧ�б���

��1��ŨH2SO4��ľ̿�ڼ���ʱ������Ӧ�Ļ�ѧ����ʽ�ǣ�
2H2SO4��Ũ��+C
CO2��+2H2O+2SO2��
| ||
2H2SO4��Ũ��+C
CO2��+2H2O+2SO2��
��
| ||
��2��ѡ�õ�ʵ��װ�ú�����˳����
�٢ߢڢۢݢ�
�٢ߢڢۢݢ�
����������������˳����װ����ţ�����3��֤����CO2���ɵ�ʵ��������
����KMnO4��Һ����ɫ�����dz�������в�����ɫ����
����KMnO4��Һ����ɫ�����dz�������в�����ɫ����
����4��װ�â�Ҳ��������Ũ������ľ̿����ʱ������Ӧ����Ӧ�������������A����ͼ2��ʾ�ı仯���ش��������⣺
������C��
NO
NO
����ҺB�е�������HNO3
HNO3
���û�ѧʽ��ʾ������B��ϡ��Һ��������ʷ�Ӧ������ɫ��Һ�����ӷ���ʽ�ǣ�


�������ת�Ƶķ����������B�ڷ�Ӧ�б���
�����Ժ�����
�����Ժ�����
����������1��ľ̿����Ũ���ᷴӦ����SO2��CO2��
��2���������ˮ������ˮ����ͭ���������������Ʒ����Һ�����Ը��������Һ�������������ݴ˼��ɽ�𣻣�3���������������̼����ʹ����ʯ��ˮ����ǣ������ø��������Һ��ȥSO2�����ͨ������ʯ��ˮ����CO2����4����������������ԣ��ܽ�����̼���������ᱻ��ԭ�����ɶ��������Ͷ�����̼��ˮ�����ͼ2���н��
����������ˮ��Ӧ���������һ������������B��ϡ��ҺΪϡ���ᣬB��ϡ��Һ��������ʷ�Ӧ������ɫ��Һ����ɫ��ҺΪ����ͭ�����ԣ��÷�ӦΪͭ�����ᷴӦ������������ԭ��Ӧ����ʽ�����ת�Ƶķ������Ŀ��
��2���������ˮ������ˮ����ͭ���������������Ʒ����Һ�����Ը��������Һ�������������ݴ˼��ɽ�𣻣�3���������������̼����ʹ����ʯ��ˮ����ǣ������ø��������Һ��ȥSO2�����ͨ������ʯ��ˮ����CO2����4����������������ԣ��ܽ�����̼���������ᱻ��ԭ�����ɶ��������Ͷ�����̼��ˮ�����ͼ2���н��
����������ˮ��Ӧ���������һ������������B��ϡ��ҺΪϡ���ᣬB��ϡ��Һ��������ʷ�Ӧ������ɫ��Һ����ɫ��ҺΪ����ͭ�����ԣ��÷�ӦΪͭ�����ᷴӦ������������ԭ��Ӧ����ʽ�����ת�Ƶķ������Ŀ��
����⣺��1��ľ̿����Ũ���ᷴӦ��̼�۱�Ũ����������Ũ���ᱻ��ԭ������ʽΪ��2H2SO4��Ũ��+C
CO2��+2H2O+2SO2����
�ʴ�Ϊ��2H2SO4��Ũ��+C
CO2��+2H2O+2SO2����
��2��ľ̿����Ũ���ᷴӦ����H2O��SO2��CO2���������ˮ��ѡ�âߣ���װ��ˮ����ͭ����ˮ����ͭ����ˮ��Ӧ������ɫ����ˮ������ͭ��������������âڣ�װ��Ʒ����Һ���������������̼����ʹ����ʯ��ˮ����ǣ������ڢ�ϴ��ƿ��װ�����Ը��������Һ�����������¶��������ڢ�ϴ��ƿ��װ�г���ʯ��ˮ��Һ�������������̼����������������β����
�ʴ�Ϊ���٢ߢڢۢݢܣ�
��3��SO2�������ط���������ԭ��Ӧ��������̼�����������ط���������ԭ��Ӧ�����ø����������ȥSO2�����������Һ�Ϻ�ɫ������Һ��ɫ���ٱ�dz������ɫ��˵��SO2�Ѿ����������ͨ������ʯ��ˮ����CO2�������ʯ��ˮ�������̼���ɲ�����ˮ��̼��ƺ�ˮ������������ǣ�
�ʴ�Ϊ������KMnO4��Һ����ɫ�����dz�������в�����ɫ������
��4����Ũ������ľ̿����ʱ������Ӧ��̼�������ɶ�����̼��Ũ���ᱻ��ԭ�ɶ���������C+4HNO3��Ũ��
2H2O+CO2��+4NO2��������������ˮ��Ӧ���������һ��������3NO2+H2O�T2HNO3+NO��������������ԣ�������������������ɫ��ҺΪͭ���ӵ���ɫ��
�ʴ�Ϊ��NO��HNO3��
��������������ԣ�������������������ɫ��ҺΪͭ���ӵ���ɫ������Ϊͭ�����ᷴӦ��ͭ��0�۱�Ϊ+2�ۣ��������ͭ�������е�+5�۵ĵ�����ԭ�����һ��������������������Ժ����ԣ����ݷ�Ӧ��������Ӧ����д����Ӧ�Ļ�ѧ����ʽ��3Cu+8HN03��ϡ��=3Cu��NO3��2+2N0��+4H2O���ӷ���ʽ֪��3molͭ��0�۱�Ϊ+2�ۣ�ʧȥ6mol���ӣ�
�ʴ�Ϊ�� �������Ժ����ԣ�
�������Ժ����ԣ�
| ||
�ʴ�Ϊ��2H2SO4��Ũ��+C
| ||
��2��ľ̿����Ũ���ᷴӦ����H2O��SO2��CO2���������ˮ��ѡ�âߣ���װ��ˮ����ͭ����ˮ����ͭ����ˮ��Ӧ������ɫ����ˮ������ͭ��������������âڣ�װ��Ʒ����Һ���������������̼����ʹ����ʯ��ˮ����ǣ������ڢ�ϴ��ƿ��װ�����Ը��������Һ�����������¶��������ڢ�ϴ��ƿ��װ�г���ʯ��ˮ��Һ�������������̼����������������β����
�ʴ�Ϊ���٢ߢڢۢݢܣ�
��3��SO2�������ط���������ԭ��Ӧ��������̼�����������ط���������ԭ��Ӧ�����ø����������ȥSO2�����������Һ�Ϻ�ɫ������Һ��ɫ���ٱ�dz������ɫ��˵��SO2�Ѿ����������ͨ������ʯ��ˮ����CO2�������ʯ��ˮ�������̼���ɲ�����ˮ��̼��ƺ�ˮ������������ǣ�
�ʴ�Ϊ������KMnO4��Һ����ɫ�����dz�������в�����ɫ������
��4����Ũ������ľ̿����ʱ������Ӧ��̼�������ɶ�����̼��Ũ���ᱻ��ԭ�ɶ���������C+4HNO3��Ũ��
| ||
�ʴ�Ϊ��NO��HNO3��
��������������ԣ�������������������ɫ��ҺΪͭ���ӵ���ɫ������Ϊͭ�����ᷴӦ��ͭ��0�۱�Ϊ+2�ۣ��������ͭ�������е�+5�۵ĵ�����ԭ�����һ��������������������Ժ����ԣ����ݷ�Ӧ��������Ӧ����д����Ӧ�Ļ�ѧ����ʽ��3Cu+8HN03��ϡ��=3Cu��NO3��2+2N0��+4H2O���ӷ���ʽ֪��3molͭ��0�۱�Ϊ+2�ۣ�ʧȥ6mol���ӣ�
�ʴ�Ϊ��
 �������Ժ����ԣ�
�������Ժ����ԣ����������⿼����̼��Ũ���ᡢŨ���ᷴӦ���������ˮ��������������̼�ļ����ǽ���Ĺؼ�����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 ��ѧȫ��������ѵ��ϵ�д�
��ѧȫ��������ѵ��ϵ�д�
�����Ŀ





