��Ŀ����
��9�֣�10 mL����a mol HCl��ϡ�����10 mL����b molNa2CO3��ϡ��Һ������ͼ��ʽ�ֱ�����Է���Һ�еμӣ�������ҡ���Թܣ��Է����±�����ɸ��⣺����������������ڱ�״���²ⶨ���Ҳ�����������ˮ�е��ܽ⣩

��1�����ж�Ӧ���̵����ӷ�Ӧ����ʽ��
�� ���� ��
��2��������������������������V1��V1'��V2��V2'��V3��V3'��Ϊ0���� ��
��3��V3��V3'�Ĵ�С��ϵΪV3 V3'���á�<�� ����>�� ��=����գ�
��4����a=0��01��b=0��008����V2= mL��V2'= mL��

��1�����ж�Ӧ���̵����ӷ�Ӧ����ʽ��
�� ���� ��
��2��������������������������V1��V1'��V2��V2'��V3��V3'��Ϊ0���� ��
��3��V3��V3'�Ĵ�С��ϵΪV3 V3'���á�<�� ����>�� ��=����գ�
��4����a=0��01��b=0��008����V2= mL��V2'= mL��
��1����CO32��+H+ = HCO3����1�֣� ��CO32��+2H+ = CO2��+H2O��1�֣�
��2��V1��1�֣� ��3��=��2�֣� ��4��V2=44��8mL��2�֣���V2'=112mL��2�֣�
��2��V1��1�֣� ��3��=��2�֣� ��4��V2=44��8mL��2�֣���V2'=112mL��2�֣�
��1��̼���ƺ�����ķ�Ӧ�Ƿֲ����еģ����Ԣ١��۵����ӷ���ʽ��CO32��+H+ = HCO3������Ӧ���������ǹ����ģ����Է�Ӧ�����ӷ���ʽ��CO32��+2H+ = CO2��+H2O��
��2������̼���ƺ����ᷴӦ�Ƿֲ����еģ�������aС��b����V1��0���������0��
��3������a����2b�����Բ�����ô�μӣ����ɵ�CO2������ͬ�ģ���V3��V3'��
��4����a=0.01��b=0.008������ݷ���ʽCO32��+H+ = HCO3����HCO3��+H+ = CO2��+H2O��֪�ڷ�Ӧ�������ɵ�CO2�ǣ�0.01��0.008��mol��0.002mol����״���µ������44.8ml�����ڷ�Ӧ���и��ݷ���ʽCO32��+2H+ = CO2��+H2O��֪�����ɵ�CO2��0.01mol��2��0.005mol�����Ա�״���µ������0.005mol��22.4L/mol��0.112L��112ml��
��2������̼���ƺ����ᷴӦ�Ƿֲ����еģ�������aС��b����V1��0���������0��
��3������a����2b�����Բ�����ô�μӣ����ɵ�CO2������ͬ�ģ���V3��V3'��
��4����a=0.01��b=0.008������ݷ���ʽCO32��+H+ = HCO3����HCO3��+H+ = CO2��+H2O��֪�ڷ�Ӧ�������ɵ�CO2�ǣ�0.01��0.008��mol��0.002mol����״���µ������44.8ml�����ڷ�Ӧ���и��ݷ���ʽCO32��+2H+ = CO2��+H2O��֪�����ɵ�CO2��0.01mol��2��0.005mol�����Ա�״���µ������0.005mol��22.4L/mol��0.112L��112ml��

��ϰ��ϵ�д�
�����Ŀ
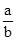 ֵ����Ϊ�� ��
ֵ����Ϊ�� ��