��Ŀ����
��5�֣���ú��Ϊȼ�Ͽ�ͨ����������;����
;���� C(s)+O2(g)=====CO2(g)����H1��0 ��
;���� ���Ƴ�ˮú����
C(s)+H2O(g)=====CO(g)+H2(g)����H2��0 ��
��ȼ��ˮú����
2CO(g)+O2(g)=====2CO2(g)����H3��0 ��
2H2(g)+O2(g)=====2H2O(g)����H4��0 ��
��ش��������⣺
(1);����ų�������������_________(����ڡ������ڡ���С�ڡ�);����ų���������
(2)��H1����H2����H3����H4����ѧ��ϵʽ��_______________��
(3)����֪���� C(s)��O2(g)��CO2(g)�� DH����393.5 kJ��mol-1
�� 2CO(g)��O2(g)��2CO2(g)�� DH��-566 kJ��mol-1
�� TiO2(s)��2Cl2(g)��TiCl4(s)��O2(g)�� DH��+141 kJ��mol-1
��TiO2(s)��2Cl2(g)��2C(s)��TiCl4(s)��2CO(g)��DH�� ��
��5�֣���1�����ڣ�1�֣� (2 ) ��H1 = ��H2 +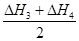 (3) ��80 kJ��mol-1
(3) ��80 kJ��mol-1
����

��ϰ��ϵ�д�
�����Ŀ