��Ŀ����
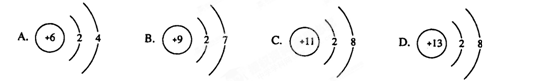
��12�֣�A��B��C��D��E���ֶ�����Ԫ�أ����ǵ�ԭ��������������Bԭ�ӵ��������������������������2����A��һ��ԭ���У���������������֮��Ϊ�㡣DԪ�ص�ԭ������������Ϊm������������Ϊn��EԪ�ص�ԭ��L�������Ϊm+n��M�������Ϊ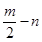 ����ش��������⣺
����ش��������⣺
��1��DԪ�������ڱ��е�λ����_____________________��
��2��д��һ��E��D�γɵĻ�������ˮ��Ӧ�����ӷ���ʽ_______________________��
��3����֪���� + H2O �� �� + ������������N��ClԪ����ɵĻ��������ӽṹģ������ͼ��ʾ��������Ư���ԡ������ClԪ�صĻ��ϼ��� ��������H2O����ͬ�ĵ����������Ļ�ѧʽΪ �� ����

��4����Dͬ�����������ڵ�Ԫ��M��N��ԭ�ӵ��Ӳ���M>N>D������Ԫ���⻯��е��ɴ�С��˳����(��д��ѧʽ) ��
��5��д��B��D�ڸ�������ȫ��Ӧ��������ĵ���ʽ___ __ ���ṹʽ___
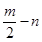 ����ش��������⣺
����ش��������⣺��1��DԪ�������ڱ��е�λ����_____________________��
��2��д��һ��E��D�γɵĻ�������ˮ��Ӧ�����ӷ���ʽ_______________________��
��3����֪���� + H2O �� �� + ������������N��ClԪ����ɵĻ��������ӽṹģ������ͼ��ʾ��������Ư���ԡ������ClԪ�صĻ��ϼ��� ��������H2O����ͬ�ĵ����������Ļ�ѧʽΪ �� ����

��4����Dͬ�����������ڵ�Ԫ��M��N��ԭ�ӵ��Ӳ���M>N>D������Ԫ���⻯��е��ɴ�С��˳����(��д��ѧʽ) ��
��5��д��B��D�ڸ�������ȫ��Ӧ��������ĵ���ʽ___ __ ���ṹʽ___
��1�����ڶ�����VIA�壨2�֣�
��2��Na2O+H2O=2Na++2OH?��2Na2O2+2H2O=4Na++4OH?+O2�� ��2�֣�
��3��+1��1�֣���NH3��1�֣�
��4��H2O>H2Se>H2S��2�֣���5��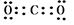 ��2�֣���O��C��O(2��)
��2�֣���O��C��O(2��)
��2��Na2O+H2O=2Na++2OH?��2Na2O2+2H2O=4Na++4OH?+O2�� ��2�֣�
��3��+1��1�֣���NH3��1�֣�
��4��H2O>H2Se>H2S��2�֣���5��
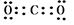 ��2�֣���O��C��O(2��)
��2�֣���O��C��O(2��)����Ԫ�صĽṹ��������ӵ��Ų����ɿ�֪��A��B��C��D��E���ֶ�����Ԫ�طֱ���H��C��N��O��Na��
��1����Ԫ��λ�ڵڶ�����VIA�塣
��2���ƺ����γɵ��������������ƻ�������ƣ���ˮ��Ӧ�����ӷ���ʽΪNa2O+H2O=2Na++2OH?��2Na2O2+2H2O=4Na++4OH?+O2����
��3�����ݷ��ӵĽṹģ�Ϳ�֪������NCl3���ṹ�����ڰ����ģ�������Ԫ�صĻ��ϼ��ǣ�1�ۡ�������Ư���ԣ�����Ǵ����ᣬ����Ϊ����H2O����ͬ�ĵ�����������Ӧ�����ǰ�����
��4������ˮ���Ӽ�������������ˮ�ķе���ͬ����Ԫ�ص��⻯���зе���ߣ���˴���H2O>H2Se>H2S��
��5��̼�����ڸ����·�Ӧ���ɵ���������CO2�����м��Լ�������ʽΪ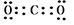 ����1�����ߴ�����ӶԵ�ʽ���ǽṹʽ������CO2�ĽṹʽΪO��C��O��
����1�����ߴ�����ӶԵ�ʽ���ǽṹʽ������CO2�ĽṹʽΪO��C��O��
��1����Ԫ��λ�ڵڶ�����VIA�塣
��2���ƺ����γɵ��������������ƻ�������ƣ���ˮ��Ӧ�����ӷ���ʽΪNa2O+H2O=2Na++2OH?��2Na2O2+2H2O=4Na++4OH?+O2����
��3�����ݷ��ӵĽṹģ�Ϳ�֪������NCl3���ṹ�����ڰ����ģ�������Ԫ�صĻ��ϼ��ǣ�1�ۡ�������Ư���ԣ�����Ǵ����ᣬ����Ϊ����H2O����ͬ�ĵ�����������Ӧ�����ǰ�����
��4������ˮ���Ӽ�������������ˮ�ķе���ͬ����Ԫ�ص��⻯���зе���ߣ���˴���H2O>H2Se>H2S��
��5��̼�����ڸ����·�Ӧ���ɵ���������CO2�����м��Լ�������ʽΪ
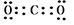 ����1�����ߴ�����ӶԵ�ʽ���ǽṹʽ������CO2�ĽṹʽΪO��C��O��
����1�����ߴ�����ӶԵ�ʽ���ǽṹʽ������CO2�ĽṹʽΪO��C��O��
��ϰ��ϵ�д�
 ��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�
��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�
�����Ŀ

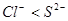 ��
�� ����
����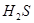 ��Ӧ����S
��Ӧ����S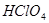 �����Ա�
�����Ա�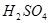 ��ǿ �ܳ�����
��ǿ �ܳ�����