��Ŀ����
����Ŀ����֪:H2(g)+I2(g)![]() 2HI(g)��H=-14.9kJ��mol-1��ij�¶��£��������Ϊ2.0L�ļס������������ܱ������г��뷴Ӧ�����ʼ���ʵ������±���ʾ�����з�Ӧ�ﵽƽ��ʱ�����c(H2)=0.008mol��L-1�������ж���ȷ���ǣ� ��
2HI(g)��H=-14.9kJ��mol-1��ij�¶��£��������Ϊ2.0L�ļס������������ܱ������г��뷴Ӧ�����ʼ���ʵ������±���ʾ�����з�Ӧ�ﵽƽ��ʱ�����c(H2)=0.008mol��L-1�������ж���ȷ���ǣ� ��
��ʼ���ʵ��� | n(H2)/mol | n(I2)/mol | n(HI)/mol |
�� | 0.02 | 0.02 | 0 |
�� | 0.04 | 0.04 | 0 |
A. ƽ��ʱ������H2��ת�����Ǽ��е�2��
B. ƽ��ʱ�����л�������ɫ��������
C. ƽ��ʱ���ס����������ı仯ֵ���
D. ���¶��£���Ӧ��ƽ�ⳣ��K=0.25
���𰸡�D
���������÷�Ӧ���ص�����Ӧǰ���������������ķ�Ӧ���������ص������º�����������ʼH2��I2��g�����ʵ���Ϊ���е�������������Ҵﵽƽ��ʱΪ��Чƽ�������ݵ�Чƽ��Ĺ��ɣ�A�����ס�����H2��ת������ȣ�����B�ƽ��ʱ����I2��g�����ʵ���Ũ��Ϊ���е����������л�������ɫ�ȼ��������C�����ת�����ʵ���Ϊ�������������зų��������Ǽ�������������D��Լ�������������ʽ��
H2��g��+I2��g��![]() 2HI��g��
2HI��g��
c����ʼ����mol/L��0.01 0.01 0
c��ת������mol/L��0.002 0.002 0.004
c��ƽ�⣩��mol/L��0.008 0.008 0.004
�÷�Ӧ��ƽ�ⳣ��ΪK=![]() =0.25����ȷ����ѡD��
=0.25����ȷ����ѡD��
�����ص�ͷ�Ӧ�ص� | ��Ч���� | ��� |
���º��� ��Ӧǰ���������������� | ��ʼͶ�ϻ������ͬ���ʱ�ʾʱ���ʵ�����Ӧ��� | ����ƽ��ʱ����ְٷֺ�����n��c��p����ͬ |
���º��� ��Ӧǰ�������������� | ��ʼͶ�ϻ������ͬ���ʱ�ʾʱ���ʵ�����Ӧ�ɱ��� | ����ƽ��ʱ����ְٷֺ�����ͬ��n��c��p�ɱ����仯 |
���º�ѹ �������������Ŀ��淴Ӧ | ��ʼͶ�ϻ������ͬ���ʱ�ʾʱ���ʵ�����Ӧ�ɱ��� | ����ƽ��ʱ����ְٷֺ�����c��ͬ��n��V�ɱ����仯 |

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ�����Ļ�������;�㷺���ش��������⣺
��1����һ�������£������ܺ�ˮ������Ӧ���ɰ���������2N2��g����6H2O��g��=4NH3��g����3O2��g����H����÷�Ӧ��صĻ�ѧ�������������£�
��ѧ�� | N��N | H��O | N��H | O=O |
E��kJ/mol�� | 946 | 463 | 391 | 496 |
��÷�Ӧ����H��________kJ��mol-1��
��2���ں����ܱ������г���2 mol N2O5��1molO2������Ӧ4NO2 (g) + O2 (g) ![]() 2N2O5 (g) ��H��
2N2O5 (g) ��H��
����֪�ڲ�ͬ�¶��²��N2O5�����ʵ�����ʱ��ı仯��ͼ��ʾ���÷�Ӧ����H_____0���������������������������������¸÷�Ӧ�������Է����У���ԭ����___________________��
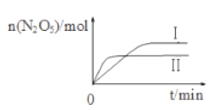
�������йظ÷�Ӧ��˵����ȷ����_______�����ţ���
A���������������ƽ�����淴Ӧ�����ƶ������������ɫ����
B�����º��ݣ��ٳ���2 mol NO2��1molO2���ٴδﵽƽ��ʱ��NO2��ת��������
C�����º��ݣ��������ڵ��ܶȱ��ֲ���ʱ����Ӧ�ﵽ��ƽ��״̬
D�����÷�Ӧ��ƽ�ⳣ��������һ���ǽ������¶�
��3��N2O5��һ��������ɫ�����������Ʊ����������⻯��ȼ�ϵ������Դ�����õ�ⷨ�Ʊ��õ�N2O5������ԭ����ͼ��ʾ�������⻯��ȼ�ϵ�صĸ�����ӦʽΪ_________��

��4��X��Y��Z��W�ֱ���HNO3��NH4NO3��NaOH��NaNO2����ǿ������е�һ�֡��±��dz�����Ũ�Ⱦ�Ϊ0��01molL��1��X��Y��Z��W��Һ��pH����X��Y��Z��1molͬʱ����ˮ�еõ������Һ��������Һ�и����ӵ�Ũ���ɴ�С��˳��Ϊ________��
0��01molL��1����Һ | X | Y | Z | W |
pH | 12 | 2 | 8.5 | 4.5 |
��5�������������������ڴ����еĺ������������ʱ���漰���·�Ӧ��
I��2NO2(g)+NaCl(s) ![]() NaNO3 (s)+ClNO(g) K1
NaNO3 (s)+ClNO(g) K1
��2NO(g)+Cl2 (g) ![]() 2CNO(g) K2
2CNO(g) K2
��4NO2��g����2NaCl��s��![]() 2NaNO3��s����2NO��g����Cl2��g����ƽ�ⳣ��K��____����K1��K2��ʾ����
2NaNO3��s����2NO��g����Cl2��g����ƽ�ⳣ��K��____����K1��K2��ʾ����
���ں��������£���2L�����ܱ������м���0��2 mol NO��0��1 mol Cl2��10minʱ��Ӧ��ﵽƽ�⣬���10min��v(ClNO)��7.5��10-3molL-1min-1����ƽ��ʱNO��ת������1=____���������������䣬��Ӧ���ں�ѹ�����½��У�ƽ��ʱNO��ת������2__��1����������������������������
����Ŀ�������й����ʼ����ʵ�������ȷ����
ѡ�� | ʵ����������� | ʵ����� |
A | ��ij��Һ�м��������ữ���Ȼ�����Һ���а�ɫ�������� | ����Һ��һ������ |
B | ��ij��Һ�м���2��KSCN��Һ����Һ���Ժ�ɫ��������Һ�м��뼸�����Ƶ���ˮ����Һ��Ϊ��ɫ | ����Һ��һ������ |
C | ��ij����ͨ��Ʒ����Һ�У�Ʒ����Һ��ɫ | ������һ��Ϊ |
D | �������л���μӵ�����������ͭ��Һ�У������δ����ɫ�������� | ���л��ﲻ��ȩ�� |
A. AB. BC. CD. D


