��Ŀ����
��ѧ��Ӧ�ڹ�ũҵ������������Ҫ��Ӧ�á���Ҫ��ش��������⣺
(1)���û�ѧ��Ӧ�����Ʊ��������ʡ�
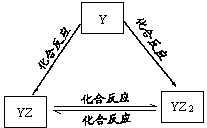
��ʵ������ͭ�Ʊ�NO�����ӷ���ʽΪ___________________��
�ڿ���Al��Fe2O3��Fe���÷�Ӧ�Ļ�ѧ����ʽΪ___________________��
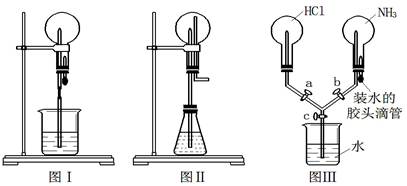
�ۺ�ˮ��������У���Ũ���ĺ�ˮ��ͨ�������������ȿ����������ɵ��壬Ȼ����̼������Һ�����壬���绯ΪBr����BrO3������������Ӧ�����ӷ���ʽ�ֱ�Ϊ__________��___________��
(2)��ѧ��Ӧ��Ϊ�������������ṩ��Դ��
���ɷ�ӦCH4 +2O2 CO2 +2H2O��������Ƴ���NaOH��ҺΪ�������Һ��ȼ�ϵ�أ��õ�ع���ʱ�����ĵ缫��ӦʽΪ��______________��
CO2 +2H2O��������Ƴ���NaOH��ҺΪ�������Һ��ȼ�ϵ�أ��õ�ع���ʱ�����ĵ缫��ӦʽΪ��______________��
��2011��ɽ���߿���ѧ�����ᵽ������ܵ�أ���ͼ�Ǹõ�صĽṹʾ��ͼ���õ�صĹ����¶�Ϊ320�����ң���ط�ӦΪ2Na+xS��Na2Sx���õ�ظ���Ϊ________(�ѧʽ)�������ĵ缫��ӦʽΪ ���øõ������Դ���д�ͭ����ʱ�����õ�64g��ͭʱ�������ϸõ�ظ������ĵ�����Ϊ_____g��
(1)��3Cu+8H++2NO3����3Cu2++2NO��+4H2O
��2Al+Fe2O3 2Fe+Al2O3��(ȱ��������1��)
2Fe+Al2O3��(ȱ��������1��)
��2Br��+Cl2��2Cl��+Br2��3Br2+3CO32����5Br��+BrO3��+3CO2��
(2)��CH4+10OH����8e����CO32��+7H2O����Na��xS+2e����Sx2����46
�������������(1)���������ǿ�����ԣ��ܺͽ���ͭ����������ԭ��Ӧ����˿�����ϡ������ͭ��Ӧ�Ʊ�NO����Ӧ�����ӷ���ʽΪ3Cu+8H++2NO3����3Cu2++2NO��+4H2O��
�����ǻ��õĽ���������ͨ�����ȷ�Ӧұ�����������Կ���Al��Fe2O3��Fe���÷�Ӧ�Ļ�ѧ����ʽΪ2Al+Fe2O3 2Fe+Al2O3��
2Fe+Al2O3��
������������ǿ�ڵ�����ģ����������ܰ��������������ɵ����壬��Ӧ�����ӷ���ʽΪ2Br��+Cl2��2Cl��+Br2������������ˮ�����ԣ��ܺ�̼������Һ�����绯��Ӧ����NaBr��NaBrO3��ˮ����Ӧ�����ӷ���ʽΪ3Br2+3CO32����5Br��+BrO3��+3CO2����
��2����ԭ����и���ʧȥ���ӣ�����������Ӧ�������õ����ӷ�����ԭ��Ӧ������ݷ�ӦCH4 +2O2 CO2 +2H2O��֪����ԭ���Ǽ��飬�����������������������Ƴ�ԭ��أ�������������ͨ�룬�����ڸ���ͨ�롣���ڵ������Һ������������Һ�����Ը����缫��ӦʽΪCH4+10OH����8e����CO32��+7H2O��
CO2 +2H2O��֪����ԭ���Ǽ��飬�����������������������Ƴ�ԭ��أ�������������ͨ�룬�����ڸ���ͨ�롣���ڵ������Һ������������Һ�����Ը����缫��ӦʽΪCH4+10OH����8e����CO32��+7H2O��
��ԭ����и���ʧȥ���ӣ�����������Ӧ�������õ����ӷ�����ԭ��Ӧ������ݵ�ط�ӦΪ2Na+xS��Na2Sx��֪�����ǻ�ԭ�������Ըõ�ظ���ΪNa��������S�õ����ӣ��������缫��ӦʽΪxS+2e����Sx2����64gͭ�����ʵ�����64g��64g/mol��1mol�����ݵ缫��ӦʽCu2����2e����Cu��֪��ת��2mol���ӣ����Ը��ݵ����غ��֪���������Ľ����Ƶ����ʵ�����2mol��������2mol��23g/mol��46g��
���㣺�������ʵ����ʡ�����ʽ����д���绯ѧԭ�����й��жϡ�Ӧ�������

 ��У����ϵ�д�
��У����ϵ�д�A��B��C��D��ԭ��������������Ķ���������Ԫ�أ�A��C��Ԫ�����ڱ��е����λ����ͼ��AԪ��������������ϵĵ�����֮��Ϊ3��BΪ�ؿ��к������Ľ���Ԫ�ء�
| A | |
| | C |
��1��Dԭ�ӽṹʾ��ͼΪ_____________��
��2����C�ĵͼ�̬�������ͨ�뵽D���ʵ�ˮ��Һ��ʹ֮��ɫ�������˼�________�ԣ�д���÷�Ӧ�����ӷ���ʽ_____________________��
��3��A������������Ӧ��ˮ�������ң��ֽ�����Cu���뵽100 mL 8.0 mol/L�ҵ�Ũ��Һ�У���ַ�Ӧ�����ռ���6.72L����״�������壬�������ijɷ���_______________����ԭ��ʧ������Ϊ_________________��
��4��������������B���ʷֱ���뵽�������Ũ�ȵ������NaOH��Һ�У���ַ�Ӧ��������������Ϊ____________________��������Ӧ�����õ���Һ��ϣ������ɰ�ɫ������������Ӧ�����ӷ���ʽΪ___________________________________________________��B���ʱ��������Ĥ����NaOH��Һ��ȥ��д���÷�Ӧ�Ļ�ѧ����ʽ____________________________________________________________��
ijԪ����ۺ�����Ļ�ѧʽ��H2RO4����Ԫ�ص���̬�⻯�ﻯѧʽ�ɱ�ʾΪ
| A��HR | B��H2R | C��RH3 | D������ȷ�� |
 ��X�е�һ�֡�
��X�е�һ�֡� C��CH3COO D��SiO
C��CH3COO D��SiO