��Ŀ����
2�����ࡢ��֬�������ʶ�����������Ӫ�����ʣ���1����֬�������������ø��������ˮ��Ϊ��֬������ͣ�д���ƣ����������������ɶ�����̼��ˮ���ṩ����������Ϊ�ϳ����������������ʵ�ԭ�ϣ�
��2������������ɵ����ʵĻ����ṹ��Ԫ���������һ�����еĹ������ǰ�����-NH2����-COOH��д�ṹ��ʽ�����ƣ��������й��ж�ʮ���ְ����ᣬ���������������ܣ���ܡ����ܡ����ϳɵİ������Ϊ������谱���ᣮ
��3�������ڵ���ø������������ˮ��Ϊ�����ǣ�C6H12O6�������������������ڱ��������ɶ�����̼��ˮ��д�������������ڱ������Ļ�ѧ����ʽ��C6H12O6+6O2$\frac{\underline{\;����\;}}{\;}$6CO2+6H2O��
��4����������һ���������ܷ������ԣ��Ӷ�ʧȥ�������ԣ���һ�������ͭ��Һ����ʱӦ�����������Ĵ������ȴ�����ţ�̣������壩������ˮ��
���� ��1����֬�Ǹ�֬�����������
��2��������һ�����еĹ������ǰ�����-NH2�����Ȼ���-COOH�������谱����ָ���������������ܺϳɻ�ϳ��ٶȲ�������������Ҫ�������ʳ������ȡ�İ����
��3�����ݷ�Ӧ��������P�������غ㶨�ɿ�����ȷ����д��ѧ����ʽ��
��4���������ţ���к��зḻ�ĵ����ʣ�
��� �⣺��1����֬��ø��������ˮ�����ɸ�֬������ͣ��ʴ�Ϊ�����ͣ�
��2��������һ�����еĹ������ǰ�����-NH2�����Ȼ���-COOH�������谱����ָ���������������ܺϳɻ�ϳ��ٶȲ�������������Ҫ�������ʳ������ȡ�İ����ᣬ
�ʴ�Ϊ��-COOH�����ܣ�
��3�������������ڷ��������������ɶ�����̼��ˮ����Ӧ�Ļ�ѧ����ʽΪ��C6H12O6+6O2$\frac{\underline{\;����\;}}{\;}$6CO2+6H2O���ʴ�Ϊ��C6H12O6+6O2$\frac{\underline{\;����\;}}{\;}$6CO2+6H2O��
��4���������ţ���к��зḻ�ĵ����ʣ��ܹ������ؽ�����������ж����ʴ�Ϊ���ȴ�����ţ�̣������壩������ˮ��
���� ���⿼����֬�����ʡ�����������ʺ������ǵ�������Ӧ���ѶȲ���ע�����֪ʶ�Ļ��ۣ�

 ��У����ϵ�д�
��У����ϵ�д�| A�� | ��NaCl��Һͨ�磬ʹNaCl�������� | |
| B�� | NaCl�ǵ���� | |
| C�� | NaCl�ĵ��뷽��ʽ�ǣ�NaCl�TNa++Cl- | |
| D�� | NaCl��Һ�ܵ��磬����Ϊ��Һ���������ƶ������� |
| A�� | �ò�����պȡ������ˮ������pH��ֽ�ϣ�Ȼ�����ɫ�����գ��ɲⶨ������ˮ��pH | |
| B�� | ��10mL 0.1 mol/L��AgNO3��Һ�еμ�10��0.1 mol/L��NaCl��Һ���а�ɫ�������ɣ��������еμ�0.1 mol/L��KI��Һ��������Ϊ��ɫ��˵����ͬ�¶���AgCl�ܽ�ȴ���AgI���ܽ�� | |
| C�� | ��1 mL 1%��NaOH��Һ�м���2 mL 2%��CuSO4��Һ�����ټ���0.5 mL�л���Y�����ȣ�δ����ש��ɫ������˵��Y�в���ȩ�� | |
| D�� | ȡ����±����Y�ڼ�����Һ��ˮ�⣬�������ữ�����ԣ��ٵμ�AgNO3��Һ�����ɵ���ɫ������˵��±�����к�����Ԫ�� |
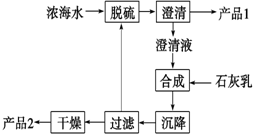 ����ˮ��������Ũ��ˮ��Դ������������ۺ����ú�ˮ����Ҫ;��֮һ��һ�����Ƚ���ˮ������õ�ˮ���ٴ�ʣ���Ũ��ˮ��ͨ��һϵ�й�����ȡ������Ʒ��
����ˮ��������Ũ��ˮ��Դ������������ۺ����ú�ˮ����Ҫ;��֮һ��һ�����Ƚ���ˮ������õ�ˮ���ٴ�ʣ���Ũ��ˮ��ͨ��һϵ�й�����ȡ������Ʒ���ش��������⣺
��1�����иĽ����Ż���ˮ�ۺ����ù��յ�������������е��Ǣڢۢܣ�����ţ���
���û�������ȡ��ˮ������߲��ֲ�Ʒ������
���Ż���ȡ��Ʒ��Ʒ�֡��ܸĽ��ء��塢þ����ȡ����
��2�����á���������������Ũ��ˮ�д���Br2�����ô������գ������������Ҫ��Ӧ��Br2+Na2CO3+H2O-��NaBr+NaBrO3+NaHCO3������1mol Br2ʱ��ת�Ƶĵ���Ϊ$\frac{5}{3}$mol��
��3����ˮ��þ��һ�ι���������ͼ��Ũ��ˮ����Ҫ�ɷ����£�
| ���� | Na+ | Mg2+ | Cl- | SO42- |
| Ũ��/��g•L-1�� | 63.7 | 28.8 | 144.6 | 46.4 |
��4������ʯī���������������������ڵ��Ȼ�þ��������Ӧ�Ļ�ѧ����ʽΪMgCl2�����ڣ�$\frac{\underline{\;���\;}}{\;}$Mg+Cl2�������ʱ����������ˮ���ڻ���ɲ�Ʒþ�����ģ�д���йط�Ӧ�Ļ�ѧ����ʽ��Mg+2H2O$\frac{\underline{\;����\;}}{\;}$Mg��OH��2+H2����
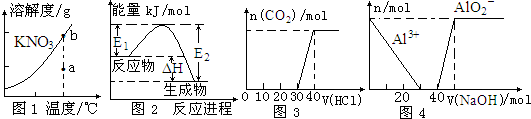
| A�� | ͼ1��ʾKNO3���ܽ�����ߣ�ͼ��a���ʾ����Һͨ�����¿��Եõ�b�� | |
| B�� | ͼ2��ʾijһ���ȷ�Ӧ����ʹ�ô���E1��E2����H���ᷢ���ı� | |
| C�� | ͼ3��ʾ��Na2CO3��NaHCO3�Ļ����Һ�еμ�ϡ����ʱ������CO2����� | |
| D�� | ͼ4��ʾ��100 mL 0.1 mol/L��AlCl3��0.1 mol/L��NH4Cl�����Һ�еμ�1 mol/L��NaOH��Һʱn��Al3+����n��AlO2-���ı仯��� |