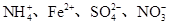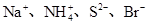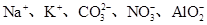��Ŀ����
ij��Һ���ܺ������������еļ���(��������Һ������H����OH��)��Na����NH4+��SO42����CO32����NO3����ȡ200 mL����Һ��������ֳ����ݣ��ֱ�������ʵ�顣
ʵ��һ����һ�ݼ��������ռ���ȣ������������ڱ�״����Ϊ224 mL��
ʵ������ڶ����ȼ������������ᣬ�������ټ�������BaCl2��Һ���ó���2.33 g��
����˵����ȷ����(����)
ʵ��һ����һ�ݼ��������ռ���ȣ������������ڱ�״����Ϊ224 mL��
ʵ������ڶ����ȼ������������ᣬ�������ټ�������BaCl2��Һ���ó���2.33 g��
����˵����ȷ����(����)
| A������Һ���ܺ���Na�� |
| B������Һһ������NH4+��SO42����CO32����NO3�� |
| C������Һһ��������NO3�� |
| D������Һһ������Na������c(Na��)��0.1 mol��L��1 |
��D
��ʵ��һ֤����Һ�к���NH4+�������ʵ���Ϊ0.01 mol��c(NH4+)��0.1 mol��L��1��ʵ������ڶ����ȼ������������ᣬ��������һ��������CO32�����ټ�������BaCl2��Һ���ù���2.33 g��֤��һ������SO42���������ʵ���Ϊ0.01 mol��c(SO42��)��0.1 mol��L��1��������Һ�ʵ����ԣ���һ������Na�����Ƿ���NO3�����жϣ�c(Na��)��0.1 mol��L��1��D��ȷ��

��ϰ��ϵ�д�
�����Ŀ
 H++HA-,HA-
H++HA-,HA-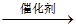 C��NO2
C��NO2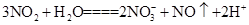
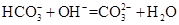
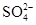 һ���ܴ�������
һ���ܴ�������