��Ŀ����
�ס��ҡ�������������������ڵ����ʼ�������ͼ��ʾ����������������ݻ���ȣ��¶���ͬ����Ӧ�мס������ݻ����䣬���е�ѹǿ���䣬��һ���¶��·�Ӧ�ﵽƽ�⡣����˵����ȷ����
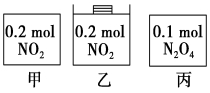
A��ƽ��ʱ��������c��NO2���Ĵ�С˳��Ϊ��>��>��
B��ƽ��ʱN2O4�İٷֺ�������>�ף���
C��ƽ��ʱ����NO2�����N2O4��ת������ͬ
D��ƽ��ʱ������ƽ����Է�����������>��>��
( 14��)
I .��1����֪H2��ȼ����285.8KJ/mol��д��Һ̬ˮ�������H2��O2���Ȼ�ѧ����ʽ ��
��2����֪2SO2(g)+O2(g) = 2SO3(g) ��H����197 kJ/mol����ͬ�¶Ⱥ�ѹǿ�����£�4molSO2��2molO2���������з�Ӧ��ƽ��ʱ�ų�������ΪQ KJ����Q 394KJ(�>����<����=��)
II.�״���һ�ֿ�������Դ�����й㷺�Ŀ�����Ӧ��ǰ������ҵ��һ������������ַ�Ӧ�ϳɼ״���
��ӦI��CO2(g)+3H2(g) CH3OH
CH3OH (g)+H2O(g) ��H1
(g)+H2O(g) ��H1
��ӦII��CO(g)+2H2(g) CH3OH(g) ��H2
CH3OH(g) ��H2
�±����������Ƿ�Ӧ���ڲ�ͬ�¶��µĻ�ѧƽ�ⳣ��(K)
�¶� | 250�� | 300�� | 350�� |
K | 2.041 | 0.270 | 0.012 |
��1���ɱ��������жϦ�H2 0(�����������������)��
��2���������ݻ����䣬���д�ʩ�����Ӽ״����ʵ��� ��
A�������¶�
B����CH3OH(g)����ϵ�з���
C��ʹ�ú��ʵĴ���
D�����º��ݳ���He��ʹ��ϵ��ѹǿ����
E����ԭ�����ٳ��� CO�� H2
��3��ij�¶��£���2 mol CO��6 mol H2����2L�ĺ����ܱ������У���ַ�Ӧ���ﵽƽ����c(CO)=0.2 mol��L-1����CO��ת����Ϊ ����ʱ���¶�Ϊ (���ϱ���ѡ��)��
��4�������£�1mol CO��nmol H2��һ���ݻ��ɱ���ܱ������з�Ӧ�ﵽƽ�������a molCH3OH������ʼʱ����3molCO+3nmolH2�����ƽ��ʱ����CH3OH_______mol��
������ʱ��pH=a�İ�ˮ��PH =b������������ϣ�ǡ����ȫ��Ӧ����ˮ����ȿɱ�ʾΪ_______________ (�ðٷ�������ʾ��
��2�������£�0.1 mol/LNaClO��Һ��pH_________0.1 mol/LNa2SO3��Һ��pH����ѡ����ڡ�����С�ڡ������ڡ�)��Ũ�Ⱦ�Ϊ0.1mol/L��Na2SO3��Na2CO3�Ļ����Һ�У�SO32-��CO32-��HSO3-��HCO3-Ũ�ȴӴ�С��˳��Ϊ ��
��֪��
H2SO3 | Kal=1.54��l0-2 | Ka2=1.02��10-7 |
HCIO | Ka1=2.95��10-8 | |
H2CO3 | Kal=4.3��10-7 | Ka2=5.6��10-11 |



 ��������Ʒ������Ʒ�ܽ⣻
��������Ʒ������Ʒ�ܽ⣻
 CO(g)+H2(g)��H>0�����c(H2O)�淴Ӧʱ��(t)�ı仯��ͼ�������ж���ȷ����
CO(g)+H2(g)��H>0�����c(H2O)�淴Ӧʱ��(t)�ı仯��ͼ�������ж���ȷ����

