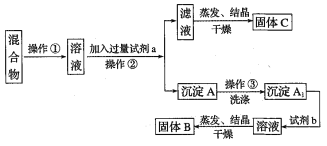
��Ŀ����
����Ŀ�����ʵ����Ǹ��л�ѧ���õ�������������������йؼ��㡣
��1����0.4 mol Al3����Al2(SO4)3��������SO42�������ʵ�����___________��
��2��___________molH2O2����ԭ������0.2molH3PO4����ԭ������ȡ�
��3��ij��������Һ�к���3.01��1022��Na+������Һ��SO42�������ʵ�����___________��
��4��0.7 mol H2O������Ϊ___________��
��5��483gNa2SO4��10H2O��������Na+�����ʵ�����___mol������H2O���ӵ���Ŀ��___����
��6��������ͬ��H2��NH3��SO2��O3���������У����з�����Ŀ���ٵ���____________��
��7��a��Xԭ�ӵ�������Ϊbg����X�����ԭ���������Ա�ʾΪ___________
��8������mgij���壬������ԭ�ӷ��ӣ���Ħ������ΪMg��mol-1���������ӵ�������NA��ʾ���������Ϸ��ż���Ӧ������д���пո�
�ٸ���������ʵ���Ϊ___________mol��
�ڸ���������ԭ������Ϊ___________����
���𰸡�0.6 mol0.40.025mol12.6g315NASO2bNA/am/M3mNA/M
��������
��1��Al2��SO4��3��n��Al3+����n��SO42-��=2��3���ʺ�0.4molAl3+��Al2��SO4��3������SO42-�����ʵ�����0.4mol��![]() =0.6mol���ʴ�Ϊ��0.6mol��
=0.6mol���ʴ�Ϊ��0.6mol��
��2��1��������Ӻ���8����ԭ�ӣ���0.2molH3PO4����1������������Ӻ���4����ԭ�ӣ�����1.6molԭ�ӵ�H2O2�����ʵ���Ϊ��![]() =0.4mol���ʴ�Ϊ��0.4��
=0.4mol���ʴ�Ϊ��0.4��
��3��ÿ�������ƻ�ѧʽ����������Ӻ������ӵĸ���֮��Ϊ1��2������3.01��1023��Na+�������Һ��SO42-�ĸ���Ϊ![]() ��3.01��1023������������ӵ����ʵ���n=
��3.01��1023������������ӵ����ʵ���n=![]() =0.25mol���ʴ�Ϊ��0.25mol��
=0.25mol���ʴ�Ϊ��0.25mol��
��4��0.7molˮ������Ϊ��18g/mol��0.7mol=12.6g���ʴ�Ϊ��12.6g��
��5��483gNa2SO410H2O�����ʵ���=![]() =1.5mol��Na+�����ʵ���ΪNa2SO410H2O��2��Ϊ1.5mol��2=3mol��H2O���ӵ����ʵ���ΪNa2SO410H2O��10��Ϊ1.5mol��10=15mol��������ˮ������ĿΪ15NA���ʴ�Ϊ��3��15NA��
=1.5mol��Na+�����ʵ���ΪNa2SO410H2O��2��Ϊ1.5mol��2=3mol��H2O���ӵ����ʵ���ΪNa2SO410H2O��10��Ϊ1.5mol��10=15mol��������ˮ������ĿΪ15NA���ʴ�Ϊ��3��15NA��
��6������n=![]() ��N=nNA��֪��������ȣ�Ħ������Խ����������ʵ���ԽС�����еķ�����Խ�٣����������У�Ħ�����������Ƕ����������Ժ��з��������ٵ��Ƕ������ʴ�Ϊ��SO2��
��N=nNA��֪��������ȣ�Ħ������Խ����������ʵ���ԽС�����еķ�����Խ�٣����������У�Ħ�����������Ƕ����������Ժ��з��������ٵ��Ƕ������ʴ�Ϊ��SO2��
��7��a��Xԭ�ӵ�������Ϊbg����NA��ԭ�ӵ�����ΪNA��![]() g����ֵ�ϵ��������ԭ���������ʸ�ԭ�����ԭ������Ϊ
g����ֵ�ϵ��������ԭ���������ʸ�ԭ�����ԭ������Ϊ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
(8)��m gij��������ʵ���Ϊ![]() =
=![]() mol���ʴ�Ϊ��
mol���ʴ�Ϊ��![]() ��
��
����Ϊһ�������к�����ԭ�ӣ����Ժ��е�ԭ����Ϊ��������2������Ϊ3��![]() mol��NAmol-1=
mol��NAmol-1=![]() NA���ʴ�Ϊ��
NA���ʴ�Ϊ��![]() NA��
NA��

 Сѧ��ʱ��ҵȫͨ����ϵ�д�
Сѧ��ʱ��ҵȫͨ����ϵ�д�����Ŀ��ijʵ��С��Ϊ��̽��SO2�����ʣ����������װ�ã�
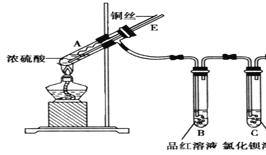
ʵ�鲽�裺
�������Ӻ�װ�ã���������ԣ��ټ����Լ���
������A�Թ���
����ͭ˿���ϳ鶯�뿪Һ�档
��1��A�Թ��з�����Ӧ�Ļ�ѧ����ʽ��______��
��2��B�Թ��е�������______��
��3���Թ�C����������ijС��ȡһ���ַ�Ӧ�����Һ���ֱ�μ������Լ�������Ԥ���ܷ����ɳ����������ɳ�����д�����ɳ����Ļ�ѧʽ��
�����Լ� | �ܷ����ɳ��� | �����Ļ�ѧʽ |
��ˮ | _____________ | __________ |
��ˮ | __________ | ___________ |