��Ŀ����
����ʵ���ܹ��ﵽԤ��Ŀ�ĵ���A.��һ֧ʢ��2 mL 2% CuSO4��Һ���Թ��У����뼸��10%��NaOH��Һ���ټ���1 mL��ȩ��Һ����к���Կ�����ɫ������ͭ��������
B.������������������ˮ��ϲ���ˮԡ���ȣ����ɵõ�ˮ������Ҵ�������
C.��һ֧�Թ��е���10�������飬�ټ���1 mL 5%��NaOH��Һ�����Ⱥ�μ���������Һ���ɹ۲쵽��dz��ɫ�廯����������
D.��ij��Һ�м����������ᣬ���κ����������ټ���BaCl2��Һ�����ְ�ɫ������֤������Һ�д���SO![]()
������A��Ӧ��NaOH��Һ�м�CuSO4��ʹ����Cu��OH��2�ʼ��ԣ�B��ȱ������H������C�й��Ⱥ�δ��HNO3�ữ��
�𰸣�D

��ϰ��ϵ�д�
 �������Ͽ��㱾ϵ�д�
�������Ͽ��㱾ϵ�д�
�����Ŀ
����ʵ���ܹ��ﵽԤ��Ŀ���ǣ�������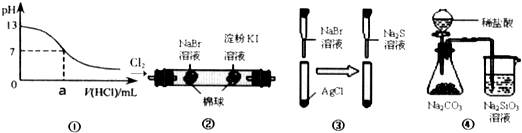

| A���ٿ����ڱ�ʾ��0.1mol?L-1��NaOH��Һ�ζ�����ĵζ����� | B���ڿ�����֤���������ԣ�Cl2��Br2��I2 | C���ۿ�����֤����Ksp��AgCl����Kxp��AgBr����Ksp��Ag2S�� | D���ܿ�����֤�����ǽ����ԣ�Cl��C��Si |