��Ŀ����
����Ŀ���к��ȵIJⶨ�Ǹ�����Ҫ�Ķ���ʵ�顣ȡ0.55mol/L��NaOH��Һ50mL��0.25mol/L������50mL����ͼ��ʾ��װ���н����к��ȵIJⶨʵ�飬�ش��������⣺

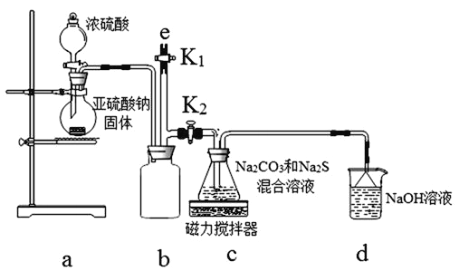
��1������ͼʵ��װ�ÿ���������ȱ�ٵ�һ�ֲ�����Ʒ��__________________________��
��2��������60mL 0.25mol��L-1H2SO4��50mL 0.55mol��L-1NaOH��Һ���з�Ӧ������ʵ����ȣ����ų�������___________�����ȡ���������ȡ�������ʵ���������ȷ���������к���__________���ȡ�������ȡ�����
��3������NaOH��Һ����ȷ�����ǣ�________�� (������ѡ��)��
A���ز������������롡 B���������������� C��һ��Ѹ�ٵ���
��4��ʹ������NaOH��Һ��Ͼ��ȵ���ȷ�����ǣ�________�� (������ѡ��)��
A�����¶ȼ�С�Ľ��� B���ҿ�ӲֽƬ�ò���������
C����������ձ� D���������¶ȼ��ϵĻ��β������������ؽ���
��5��ʵ���������±���������д�±��еĿհף�
�¶� ʵ������� | ��ʼ�¶�t1�� | ��ֹ�¶�t2/�� | �¶Ȳ�ƽ��ֵ (t2��t1)/�� | ||
H2SO4 | NaOH | ƽ��ֵ | |||
1 | 26.2 | 26.0 | 26.1 | 29.5 | ______ |
2 | 27.0 | 27.4 | 27.2 | 32.3 | |
3 | 25.9 | 25.9 | 25.9 | 29.2 | |
4 | 26.4 | 26.2 | 26.3 | 29.8 | |
�ڽ�����Ϊ0.55 mol/L NaOH��Һ��0.25 mol/L������Һ���ܶȶ���1 g/cm3���кͺ�������Һ�ı�����c��4.18 J/(g����)�����к�����H��___________ ( ȡС�����һλ)��
������ʵ����ֵ�����57.3 kJ/mol��ƫ�����ƫ���ԭ�������(����ĸ)_________��
a��ʵ��װ�ñ��¡�����Ч����
b�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶�
c���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ���
���𰸡����β����������������CD3.4-56.8kJ/mola b c
��������
��1�������ȼƵĹ������֪����װ�õ�ȱ�������ǻ��β������������˱�����������β����������
��2����Ӧ�ų����������������Լ�������Ķ����йأ�����60mL0.25molL-1H2SO4��50mL0.55molL-1NaOH��Һ���з�Ӧ������ʵ����ȣ�����ˮ�������࣬���ų�������ƫ�ߣ������к��ȵľ���ǿ���ǿ�Ӧ����1molˮʱ�ų����ȣ��к�����ȣ���ˣ�������ȷ���ǣ�����ȣ������
��3���к��ȵIJⶨ�У����뾡����������ɢʧ�����Ե�������������Һʱ������һ�β���Ѹ�ٵ����ձ��У�����Cѡ������ȷ�ģ����������C��
��4��A���¶ȼ����ڲⶨ�¶ȣ�����ʹ���¶ȼƽ�����Һ����A����
B���ҿ�ӲֽƬ�ò��������裬�ᵼ������ɢʧ��Ӱ��ⶨ�������B����
C����������ձ����ᵼ����Һ������������������ɢʧ��Ӱ��ⶨ�������C����
D���������¶ȼ��ϵĻ��β���������ؽ���������ʹ���������������Һ��Ͼ��ȣ��ֿ��Լ�������ɢʧ������Dѡ������ȷ�ģ�
�������������������D��
��5�����Ĵβⶨ�¶Ȳ�ֱ�Ϊ3.4�棬5.1�棬3.3�棬3.5�������е�2�ε��¶Ȳ����ϴ�Ӧ�����������������¶Ȳ��ƽ��ֵΪ����3.4+3.3+3.5��/3=3.4������˱�����ǣ�3.4��
��50mL0.55mol/L����������50mL0.25mol/L������Һ�����кͷ�Ӧ������ˮ�����ʵ���Ϊ0.05L��0.50mol/L=0.025mol����Һ������Ϊ100mL��1g/cm3=100g���¶ȱ仯��ֵΪ��T=3.4����������0.025molˮ�ų�������Ϊ��Q=mc��T=100g��4.18J/��g�棩��3.4��=1421.2J����1.4212KJ������ʵ���õ��к�����H=-1.4212/0.025=-56.8 kJ/mol����ˣ�������ȷ���ǣ�-56.8kJ/mol��
��a��ʵ��װ�ñ��¡�����Ч������ã��������������ʧ���ⶨ���ƫС����a��ȷ��
b���¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�Ӳ���ϡH2SO4���¶ȣ���Ϊ�¶ȼ��ϻ����������ƣ��������������ᷴӦ���ȣ������������ʼ�¶�ƫ�ߣ��¶Ȳ�ƫС���ⶨ���ƫС����b��ȷ��
c������һ�ο��ٽ�NaOH��Һ����ʢ�������С�ձ��У��������ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ��У�����ᵼ�½϶�����ɢʧ����c��ȷ��
��ˣ�������ȷ���ǣ�abc��

 ʱ�����������ҵԭ���ܳ�����ϵ�д�
ʱ�����������ҵԭ���ܳ�����ϵ�д� ����νӽ̲���ĩ���Ԥϰ�人������ϵ�д�
����νӽ̲���ĩ���Ԥϰ�人������ϵ�д� ������ҵ��ٳɳ����½������������ϵ�д�
������ҵ��ٳɳ����½������������ϵ�д�