��Ŀ����
 ��Ȼ�������һ�ְ���ʯ�Ŀ�ʯ���仯ѧʽ��xCaCO3?yMgCO3������Ϊԭ�ϣ�����ȡ�ͻ���ϵȣ�
��Ȼ�������һ�ְ���ʯ�Ŀ�ʯ���仯ѧʽ��xCaCO3?yMgCO3������Ϊԭ�ϣ�����ȡ�ͻ���ϵȣ���1����ȡ27.6g����ʯ�����ȵ��������ٱ仯�����������ͻ����MgO����mol������x��y�Ĵ���ʽ��ʾ��
��2������������Ӧ�У��ռ���CO26.72L����״���£�����д������ʯ�Ļ�ѧʽ��ȡx��y����С�������ȣ���
��3������ȡ����ʯmg������������̿�ۻ�ϣ������ض������и�������ǿ��һ��ʱ���ð���ʯ�ֽ���Ϊa������һ����̼�����ΪVL����״�������Լ��㣬V=
0.73ma
0.73ma
����m��a����ʽ��ʾ������֪CaO+3C
| ���� |
2MgO+5C
| ���� |
CO2+C
| ���� |
����ͼ�л���V��a�Ĺ�ϵͼ��
��������1��̼��ơ�̼��þ�ڸ����¿ɷֽ����������ơ�����þ�Ͷ�����̼������������ƶϳ�����ʯ����ʱ�ֽ����������ơ�����þ�����������̼������Mgԭ���غ����MgO�����ʵ�����
��2�������������̼��������д����Ӧ�Ļ�ѧ����ʽ�������÷�Ӧ�а���ʯ�������̼��������ϵ���������ѧʽ��x��y�ıȣ�Ȼ��ȡ��С������д������ʯ�Ļ�ѧʽ��
��3�������ڸ�������ʱ��ǿ�ȣ�����ʯ�ֽ���Ķ�����̼���������̿�۷�����Ӧ����Ӧ����һ����̼�����û�ѧ��Ӧ����ʽ�ҳ�����ʯ��CO�ù�ϵ���ݴ������
��2�������������̼��������д����Ӧ�Ļ�ѧ����ʽ�������÷�Ӧ�а���ʯ�������̼��������ϵ���������ѧʽ��x��y�ıȣ�Ȼ��ȡ��С������д������ʯ�Ļ�ѧʽ��
��3�������ڸ�������ʱ��ǿ�ȣ�����ʯ�ֽ���Ķ�����̼���������̿�۷�����Ӧ����Ӧ����һ����̼�����û�ѧ��Ӧ����ʽ�ҳ�����ʯ��CO�ù�ϵ���ݴ������
����⣺��1�������ͻ����MgO�����ʵ���Ϊn��MgO��=n��Mg��=
��y=
mol��
�������ͻ����MgO�����ʵ���Ϊ
mol��
��2���������⣬����ʯ���ȷֽ�Ļ�ѧ����ʽΪ��
������̼������Ϊ
��44g/mol=1.32g��
xCaCO3?yMgCO3
xCaO+yMgO+��x+y��CO2��
100x+84y 44��x+y��
2.76g 1.32g
����
=
��
��� x��y=1��1��
��˰���ʯ�Ļ�ѧʽΪCaCO3?MgCO3��
�𣺰���ʯ�Ļ�ѧʽΪCaCO3?MgCO3��
��3����CaCO3?MgCO3
CaO+MgO+2CO2����
CaO+3C
CaC2+CO
2MgO+5C
Mg2C3+2CO
CO2+C
2CO�����֪
CaO��CO MgO��CO CO2��2CO
������CaCO3?MgCO3��6CO
184 6��28
mg��a
��28g/mol=1.25Vg
����
=
��
���V=0.73ma��
�ʴ�Ϊ��0.73ma��
��V=0.73ma��֪��V��a�Ĺ�ϵ������ͼ��Ϊб��Ϊ2.02����������ͼ���ϵ��
��V��a�Ĺ�ϵͼΪ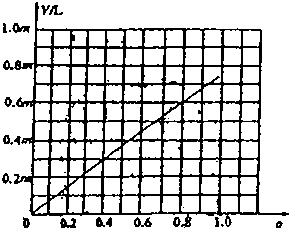 ��
��
| 27.6g |
| (100x+84y)g/mol |
| 27.6y |
| 100x+84y |
�������ͻ����MgO�����ʵ���Ϊ
| 27.6y |
| 100x+84y |
��2���������⣬����ʯ���ȷֽ�Ļ�ѧ����ʽΪ��
������̼������Ϊ
| 6.72L |
| 22.4L/mol |
xCaCO3?yMgCO3
| ||
100x+84y 44��x+y��
2.76g 1.32g
����
| 100x+84y |
| 2.76g |
| 44(x+y) |
| 1.32g |
��� x��y=1��1��
��˰���ʯ�Ļ�ѧʽΪCaCO3?MgCO3��
�𣺰���ʯ�Ļ�ѧʽΪCaCO3?MgCO3��
��3����CaCO3?MgCO3
| ||
CaO+3C
| ���� |
2MgO+5C
| ���� |
CO2+C
| ||
CaO��CO MgO��CO CO2��2CO
������CaCO3?MgCO3��6CO
184 6��28
mg��a
| VL |
| 22.4L/mol |
����
| 184 |
| mag |
| 6��28 |
| 1.25Vg |
���V=0.73ma��
�ʴ�Ϊ��0.73ma��
��V=0.73ma��֪��V��a�Ĺ�ϵ������ͼ��Ϊб��Ϊ2.02����������ͼ���ϵ��
��V��a�Ĺ�ϵͼΪ
 ��
��������������ѣ�����ѧ�����û�ѧ��Ӧ����ʽ�ļ��㣬���Ĺؼ������ü�����ѧ��Ӧ�ó���ϵʽ�����ù�ϵʽ������������һ����̼�Ĺ�ϵ��ע���˽��ⷽ����Ӧ�ã�

��ϰ��ϵ�д�
 ���ĺ����Ͼ�������ϵ�д�
���ĺ����Ͼ�������ϵ�д�
�����Ŀ
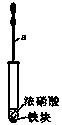 ij��ѧѧϰС������ͼ��ʾװ���о���ͬ�������������ᷴӦ���������ʵ�鲽�����£�
ij��ѧѧϰС������ͼ��ʾװ���о���ͬ�������������ᷴӦ���������ʵ�鲽�����£�
 CaC2+CO
CaC2+CO Mg2C3+2CO
Mg2C3+2CO 2CO��
2CO��