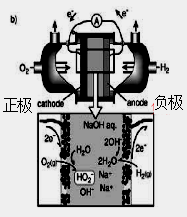
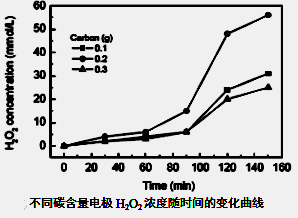
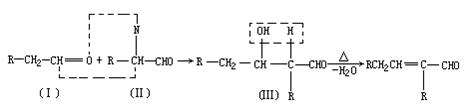
��Ŀ����
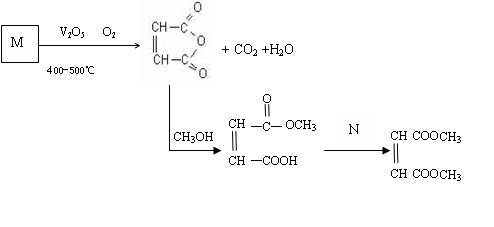
˳��ϩ�����������һ����Ҫ���л�����ԭ�ϣ��㷺Ӧ����Ϳ�ϣ����ᣬɱ�����������ˮ�������ȷ��档����������ʯ�Ͳ�ƷM�ϳ�˳��ϩ����������IJ��ֹ���
 ��1����֪��M����Է�������Ϊ78�������ں�̼��Ϊ92.3��������C��H����̼̼��������ͬ��������M�Ļ�ѧʽΪ
��1����֪��M����Է�������Ϊ78�������ں�̼��Ϊ92.3��������C��H����̼̼��������ͬ��������M�Ļ�ѧʽΪ
��2�������м����A���еĹ�����Ϊ (������)
��3�������������IJ�Ʒ�еõ���˳��ϩ�����������Ʒ�����ȡ�90%��������˳��ϩ�����������ѧ�������ȡ�98.5%���ʹֲ�Ʒ�ķ���
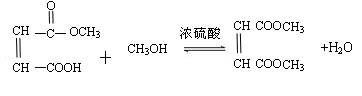
(4)д�����м����A�ϳ�˳��ϩ����������ķ���ʽ
��5���м����A��������H2��Ӧ�ɵ��л���B��C4H10O4����д��B��������Ŀ��ܽṹ��ʽ
|
 ��1����֪��M����Է�������Ϊ78�������ں�̼��Ϊ92.3��������C��H����̼̼��������ͬ��������M�Ļ�ѧʽΪ
��1����֪��M����Է�������Ϊ78�������ں�̼��Ϊ92.3��������C��H����̼̼��������ͬ��������M�Ļ�ѧʽΪ ��2�������м����A���еĹ�����Ϊ (������)
��3�������������IJ�Ʒ�еõ���˳��ϩ�����������Ʒ�����ȡ�90%��������˳��ϩ�����������ѧ�������ȡ�98.5%���ʹֲ�Ʒ�ķ���
(4)д�����м����A�ϳ�˳��ϩ����������ķ���ʽ
��5���м����A��������H2��Ӧ�ɵ��л���B��C4H10O4����д��B��������Ŀ��ܽṹ��ʽ
(1)C6H6(2)̼̼˫�� ����(3)�ֱ�ȡ������Ʒ�ʹ�Ʒ���Թܣ��ֱ����NaHCO3��Һ�����Թ��������ݲ�������ԭ�Լ�Ϊ��Ʒ��
(4)
(5)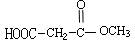 ��
��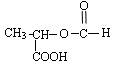
(4)

(5)
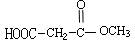 ��
��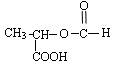
���⿼���л��ϳɡ���1����M��̼������Ϊ78��92.3��=72��̼ԭ�Ӹ���Ϊ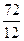 =6��Ȼ��ͨʽCxHy�����ԭ�Ӹ���Ϊ6��M�ķ���ʽΪC6H6����3�����������֪����Ʒ�ʹ�Ʒ���������ڴ�Ʒ�к����Ȼ�����˳��ϩ�����������ѧ����ֻ�����������Կ��������Ȼ���NaHCO3��Ӧ�������������飻��4��NΪ�״�����Ϊ�״����м����A����������Ӧ����5��
=6��Ȼ��ͨʽCxHy�����ԭ�Ӹ���Ϊ6��M�ķ���ʽΪC6H6����3�����������֪����Ʒ�ʹ�Ʒ���������ڴ�Ʒ�к����Ȼ�����˳��ϩ�����������ѧ����ֻ�����������Կ��������Ȼ���NaHCO3��Ӧ�������������飻��4��NΪ�״�����Ϊ�״����м����A����������Ӧ����5��
�м����A��������H2��Ӧ������B��B�ķ���ʽΪ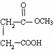 ������ṹ�����У�HCOOCH2CH2COOCH3��CH3COOCH2COOCH3��CH3CH2COOCOOCH3��HCOOCH2COOCH2CH3��CH3COOCOOCH2CH3�ȡ�
������ṹ�����У�HCOOCH2CH2COOCH3��CH3COOCH2COOCH3��CH3CH2COOCOOCH3��HCOOCH2COOCH2CH3��CH3COOCOOCH2CH3�ȡ�
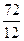 =6��Ȼ��ͨʽCxHy�����ԭ�Ӹ���Ϊ6��M�ķ���ʽΪC6H6����3�����������֪����Ʒ�ʹ�Ʒ���������ڴ�Ʒ�к����Ȼ�����˳��ϩ�����������ѧ����ֻ�����������Կ��������Ȼ���NaHCO3��Ӧ�������������飻��4��NΪ�״�����Ϊ�״����м����A����������Ӧ����5��
=6��Ȼ��ͨʽCxHy�����ԭ�Ӹ���Ϊ6��M�ķ���ʽΪC6H6����3�����������֪����Ʒ�ʹ�Ʒ���������ڴ�Ʒ�к����Ȼ�����˳��ϩ�����������ѧ����ֻ�����������Կ��������Ȼ���NaHCO3��Ӧ�������������飻��4��NΪ�״�����Ϊ�״����м����A����������Ӧ����5���м����A��������H2��Ӧ������B��B�ķ���ʽΪ
 ������ṹ�����У�HCOOCH2CH2COOCH3��CH3COOCH2COOCH3��CH3CH2COOCOOCH3��HCOOCH2COOCH2CH3��CH3COOCOOCH2CH3�ȡ�
������ṹ�����У�HCOOCH2CH2COOCH3��CH3COOCH2COOCH3��CH3CH2COOCOOCH3��HCOOCH2COOCH2CH3��CH3COOCOOCH2CH3�ȡ�
��ϰ��ϵ�д�
�����Ŀ
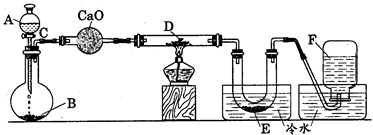
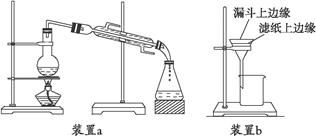
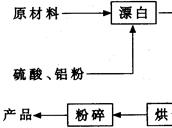
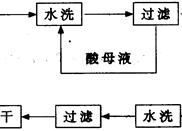
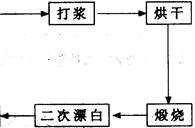
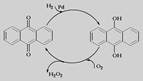

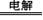 (NH4)2S2O8 + H2����
(NH4)2S2O8 + H2����