��Ŀ����
�ڹ����г�ʹ��ú��ȼ�ϣ���úȼ�պ������к���SO2������ѭ�����á���ģʽ������Ҫ��SO2�ռ�������������Ϊ���õĶ�����
��1��������й����ŷ�SO2�����γ�������Ⱦ��������ˮ��pHԼΪ______��
��2����������ͨ��Ũ��ˮ���ɵõ�һ�ַ��ϣ�д����Ӧ�Ļ�ѧ�ķ���ʽ��______
��3��ij��������Ǹ���SO2����ˮ�Ķ�����Ӧ���ⶨSO2�ĺ������÷�Ӧ�Ļ�ѧ����ʽΪ______����Ӧ����Һ��pH______����������С�����䡱������ÿ��ȡ���Ŀ���Ϊ200mL��ͨ����������ˮ�У����ƽ����2.408��1018������ת�ƣ���SO2�ĺ���Ϊ______ mg?L-1�����涨������SO2�������ó���0.02mg?L-1���������Ƿ���ϴ�����������______������ϡ������ϡ�����
��4�������������ձ������о���Na2SO3���շ���Ϊ����SO2��Ⱦ��һ���·�����
��һ������Na2SO3ˮ��Һ����SO2��
�ڶ����Ǽ�������Һ��ʹ֮��������Na2SO3��ͬʱ�õ�����Ũ��SO2ˮ��������Ʒ��д������������Ӧ�Ļ�ѧ����ʽ��
��______��
��______��
�⣺��1��������ˮ���ܽ�CO2��pHԼΪ5.6�������ܽ�SO2ʱ��PH��5.6���ʴ�Ϊ��5.6��
��2����������������������백ˮ��Ӧ��������泥���Ӧ����ʽΪSO3+2NH3?H2O=��NH4��2SO4+H2O��
�ʴ�Ϊ��SO3+2NH3?H2O=��NH4��2SO4+H2O��
��3��SO2���л�ԭ�ԣ�Br2���������ԣ����߷���������ԭ��Ӧ����Ӧ����ʽΪSO2+Br2+2H2O=2H2SO4+2HBr������HBr��H2SO4����Һ���Ա�ǿ��PH��С��
n��e-��=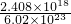 mol=4��10-6mol����n��SO2��=2��10-6mol��m��SO2��=2��10-6mol��64g/mol=1.28��10-4g��
mol=4��10-6mol����n��SO2��=2��10-6mol��m��SO2��=2��10-6mol��64g/mol=1.28��10-4g��
SO2�ĺ���Ϊ��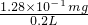 =0.64mg/L�������ϴ�������������
=0.64mg/L�������ϴ�������������
�ʴ�Ϊ��SO2+Br2+2H2O=2H2SO4+2HBr����С�� 0.64�������ϣ�
��4��Na2SO3ˮ��Һ����SO2����NaHSO3����Ӧ�ķ���ʽΪNa2SO3+SO2+H2O=2NaHSO3��NaHSO3���ȶ������ȷֽ⣬��Ӧ�ķ���ʽΪ2NaHSO3 Na2SO3+SO2��+H2O��
Na2SO3+SO2��+H2O��
�ʴ�Ϊ��Na2SO3+SO2+H2O=2NaHSO3��2NaHSO3 Na2SO3+SO2��+H2O��
Na2SO3+SO2��+H2O��
��������1��������ˮ���ܽ�CO2��pHԼΪ5.6��
��2����������������������백ˮ��Ӧ��������泥�
��3��SO2���л�ԭ�ԣ�Br2���������ԣ����߷���������ԭ��Ӧ�����ݷ���ʽ�ж���ҺPH�仯�Լ���ؼ��㣻
��4������Na2SO3��NaHSO3�����ʷ�����
���������⿼�������������ʣ���Ŀ�Ѷ��еȣ�ע����ػ���֪ʶ�Ļ��ۣ�
��2����������������������백ˮ��Ӧ��������泥���Ӧ����ʽΪSO3+2NH3?H2O=��NH4��2SO4+H2O��
�ʴ�Ϊ��SO3+2NH3?H2O=��NH4��2SO4+H2O��
��3��SO2���л�ԭ�ԣ�Br2���������ԣ����߷���������ԭ��Ӧ����Ӧ����ʽΪSO2+Br2+2H2O=2H2SO4+2HBr������HBr��H2SO4����Һ���Ա�ǿ��PH��С��
n��e-��=
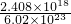 mol=4��10-6mol����n��SO2��=2��10-6mol��m��SO2��=2��10-6mol��64g/mol=1.28��10-4g��
mol=4��10-6mol����n��SO2��=2��10-6mol��m��SO2��=2��10-6mol��64g/mol=1.28��10-4g��SO2�ĺ���Ϊ��
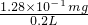 =0.64mg/L�������ϴ�������������
=0.64mg/L�������ϴ��������������ʴ�Ϊ��SO2+Br2+2H2O=2H2SO4+2HBr����С�� 0.64�������ϣ�
��4��Na2SO3ˮ��Һ����SO2����NaHSO3����Ӧ�ķ���ʽΪNa2SO3+SO2+H2O=2NaHSO3��NaHSO3���ȶ������ȷֽ⣬��Ӧ�ķ���ʽΪ2NaHSO3
 Na2SO3+SO2��+H2O��
Na2SO3+SO2��+H2O���ʴ�Ϊ��Na2SO3+SO2+H2O=2NaHSO3��2NaHSO3
 Na2SO3+SO2��+H2O��
Na2SO3+SO2��+H2O����������1��������ˮ���ܽ�CO2��pHԼΪ5.6��
��2����������������������백ˮ��Ӧ��������泥�
��3��SO2���л�ԭ�ԣ�Br2���������ԣ����߷���������ԭ��Ӧ�����ݷ���ʽ�ж���ҺPH�仯�Լ���ؼ��㣻
��4������Na2SO3��NaHSO3�����ʷ�����
���������⿼�������������ʣ���Ŀ�Ѷ��еȣ�ע����ػ���֪ʶ�Ļ��ۣ�

��ϰ��ϵ�д�
 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д� �ۺ��Բ�ϵ�д�
�ۺ��Բ�ϵ�д�
�����Ŀ
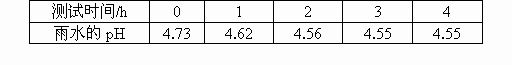
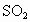 Ũ�ȣ���20���͵�60��70�����10��䣬�ɷ��糧�ŷų���
Ũ�ȣ���20���͵�60��70�����10��䣬�ɷ��糧�ŷų���