��Ŀ����
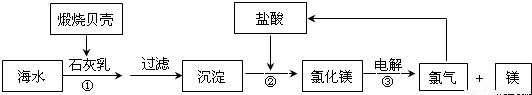
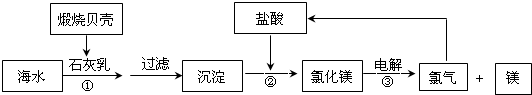
Ŀǰ��������������þ��60%���Ժ�ˮ������������ͼ��ͼ
��1�����ǵ���Ҫ��ѧ�ɷ�Ϊ
��2��д����Ӧ�ڵ����ӷ���ʽ��
��3��д��Mg��CO2��Ӧ�Ļ�ѧ����ʽ�����������ת�Ƶķ�������Ŀ��
 �����л�ԭ����
�����л�ԭ����
��4������Ȼ�þ���õ��������������������⣬��ٳ������ڹ�ҵ�ϵ���һ����;���û�ѧ����ʽ��ʾ��

��1�����ǵ���Ҫ��ѧ�ɷ�Ϊ
CaCO3
CaCO3
��д��ѧʽ������2��д����Ӧ�ڵ����ӷ���ʽ��
Mg��OH��2+2H+=Mg2++2H2O
Mg��OH��2+2H+=Mg2++2H2O
����3��д��Mg��CO2��Ӧ�Ļ�ѧ����ʽ�����������ת�Ƶķ�������Ŀ��


Mg
Mg
������ԭ��Ԫ��ΪC
C
����4������Ȼ�þ���õ��������������������⣬��ٳ������ڹ�ҵ�ϵ���һ����;���û�ѧ����ʽ��ʾ��
2Ca��OH��2+2Cl2�T2H2O+CaCl2+Ca��ClO��2
2Ca��OH��2+2Cl2�T2H2O+CaCl2+Ca��ClO��2
����������1�����ǵ���Ҫ��ѧ�ɷ�Ϊ̼��ƣ�
��2����������ͼ��֪��Ӧ����������þ���������ᷴӦ��
��3����������ԭ��Ӧ�У����ϼ�����Ԫ��ʧ���ӣ����ϼ۽���Ԫ�صõ����ӣ���ʧ��������ȼ�Ϊת�Ƶ����������ϼ۽���Ԫ���ڷ�Ӧ�б���ԭ�����ϼ�����Ԫ�����ڵķ�Ӧ���ǻ�ԭ����
��4������������ʯ������ȡƯ�ۣ�
��2����������ͼ��֪��Ӧ����������þ���������ᷴӦ��
��3����������ԭ��Ӧ�У����ϼ�����Ԫ��ʧ���ӣ����ϼ۽���Ԫ�صõ����ӣ���ʧ��������ȼ�Ϊת�Ƶ����������ϼ۽���Ԫ���ڷ�Ӧ�б���ԭ�����ϼ�����Ԫ�����ڵķ�Ӧ���ǻ�ԭ����
��4������������ʯ������ȡƯ�ۣ�
����⣺��1�����ǵ���Ҫ��ѧ�ɷ�Ϊ̼��ƣ��ʴ�Ϊ��CaCO3��
��2����Ӧ����������þ���������ᷴӦ������ʽΪ��Mg��OH��2+2HCl=MgCl2+2H2O�����ӷ���ʽΪ��Mg��OH��2+2H+=Mg2++2H2O��
�ʴ�Ϊ��Mg��OH��2+2H+=Mg2++2H2O��
��3����������ԭ��Ӧ2Mg+CO2
2Mg0+C�У�þԪ�ػ��ϼ����ߣ�������������ԭ����̼Ԫ�ػ��ϼ۽��ͣ�����ԭ��������������ʧ���������Ϊ4������ת��������£� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��Mg��C��
��Mg��C��
��4������������ʯ������ȡƯ�ۣ�����ʽΪ��2Ca��OH��2+2Cl2�T2H2O+CaCl2+Ca��ClO��2��
�ʴ�Ϊ��2Ca��OH��2+2Cl2�T2H2O+CaCl2+Ca��ClO��2��
��2����Ӧ����������þ���������ᷴӦ������ʽΪ��Mg��OH��2+2HCl=MgCl2+2H2O�����ӷ���ʽΪ��Mg��OH��2+2H+=Mg2++2H2O��
�ʴ�Ϊ��Mg��OH��2+2H+=Mg2++2H2O��
��3����������ԭ��Ӧ2Mg+CO2
| ||
 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��Mg��C��
��Mg��C����4������������ʯ������ȡƯ�ۣ�����ʽΪ��2Ca��OH��2+2Cl2�T2H2O+CaCl2+Ca��ClO��2��
�ʴ�Ϊ��2Ca��OH��2+2Cl2�T2H2O+CaCl2+Ca��ClO��2��
������������Ҫ������þ���Ʊ����̣��漰��������ԭ��Ӧ��֪ʶ��ע���Ӧ֪ʶ�Ļ��ۣ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
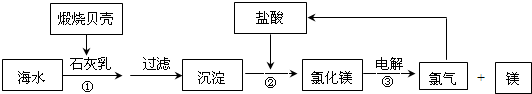
 Mg+Cl2��
Mg+Cl2�� 2Mg+O2��
2Mg+O2��