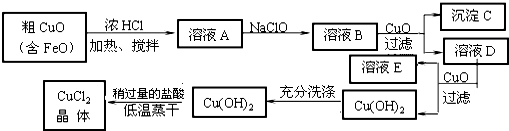
��Ŀ����
��10�֣���ҵ����ȡCuCl2�������������£�
�����±����ݣ��ش��������⣺
| �� �� | Fe(OH)2 | Cu(OH)2 | Fe(OH)3 |
| ��ȫ����ʱ��pH��Χ | ��9.6 | ��6.4 | 3��4 |
�� ��ҺA�м���NaClO��Ŀ���� ��
�˷�Ӧ���ӷ���ʽΪ ��
�� ����ҺB�м���CuO�������� ��
�� ����a��Ŀ���� ��
�� ��Cu(OH)2��������ʹCu(OH)2ת��ΪCuCl2�����ö�������͵������ɵ�Ŀ���� ��
�� ��Fe2������ΪFe3����ʹ���������ȫ
2 H+ + ClO-+2 Fe2+=2Fe3+ +Cl-+ H2O
����:��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д���10�֣���ҵ����ȡCuCl2�������������£�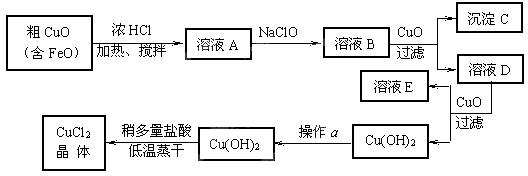
�����±����ݣ��ش��������⣺
| �� �� | Fe(OH)2 | Cu(OH)2 | Fe(OH)3 |
| ��ȫ����ʱ��pH��Χ | ��9.6 | ��6.4 | 3��4 |
�˷�Ӧ���ӷ���ʽΪ ��
�� ����ҺB�м���CuO�������� ��
�� ����a��Ŀ���� ��
�� ��Cu(OH)2��������ʹCu(OH)2ת��ΪCuCl2�����ö�������͵������ɵ�Ŀ���� ��
��10�֣���ҵ����ȡCuCl2�������������£�

�����±����ݣ��ش��������⣺
|
�� �� |
Fe(OH)2 |
Cu(OH)2 |
Fe(OH)3 |
|
��ȫ����ʱ��pH��Χ |
��9.6 |
��6.4 |
3��4 |
�� ��ҺA�м���NaClO��Ŀ���� ��
�˷�Ӧ���ӷ���ʽΪ ��
�� ����ҺB�м���CuO�������� ��
�� ����a��Ŀ���� ��[��Դ:]
�� ��Cu(OH)2��������ʹCu(OH)2ת��ΪCuCl2�����ö�������͵������ɵ�Ŀ���� ��
��8��,ÿ��2�֣���ҵ����ȡCuCl2�������������£�
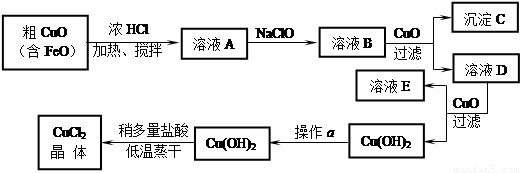
�����±����ݣ��ش��������⣺
|
�� �� |
Fe(OH)2 |
Cu(OH)2 |
Fe(OH)3 |
|
�ܶȻ���25�� |
8.0��10��16 |
2.2��10��20 |
4.0��10��38 |
|
��ȫ����ʱ��pH��Χ |
��9.6 |
��6.4 |
3��4 |
�� ��ҺA�м���NaClO��Ŀ���� ��
�� ����ҺB�м���CuO�������� ��
�� ����a��Ŀ���� ��
�� ��Cu(OH)2��������ʹCu(OH)2ת��ΪCuCl2�����ö�������͵������ɵ�Ŀ���� ��