��Ŀ����
��8�֣� һ���¶��£���2mol SO2�����1mol O2����ͨ��һ�ܱ������У��������·�Ӧ��2SO2(g)+O2 (g) 2SO3(g)������д���пհף�
2SO3(g)������д���пհף�
����������̶�Ϊ2L����Ӧ1minʱ���ʣ��1.2mol SO2��SO3��Ũ��Ϊ0.4mol/L��
��1min�ڣ�O2��ƽ����Ӧ����Ϊ __________ ��
������Ӧ��2min�ﵽƽ�⣬ƽ��ʱSO3��Ũ��_ _0.8mol/L(����ڡ��������ڡ���С�ڡ�)��
�۸ı���ʼ���ʼ����������ʹ��Ӧ�ﵽƽ��ʱSO3�����ʵ���������ԭƽ����ȣ���ʼ�������������SO2��O2 ��SO3�����ʵ���a��b��c֮��Ӧ����Ĺ�ϵʽ_______��_______��
 2SO3(g)������д���пհף�
2SO3(g)������д���пհף�����������̶�Ϊ2L����Ӧ1minʱ���ʣ��1.2mol SO2��SO3��Ũ��Ϊ0.4mol/L��
��1min�ڣ�O2��ƽ����Ӧ����Ϊ __________ ��
������Ӧ��2min�ﵽƽ�⣬ƽ��ʱSO3��Ũ��_ _0.8mol/L(����ڡ��������ڡ���С�ڡ�)��
�۸ı���ʼ���ʼ����������ʹ��Ӧ�ﵽƽ��ʱSO3�����ʵ���������ԭƽ����ȣ���ʼ�������������SO2��O2 ��SO3�����ʵ���a��b��c֮��Ӧ����Ĺ�ϵʽ_______��_______��
��1��0.2 mol��L�D1��min�D1 (2��) ��2����(2��)
��3��a+c="2 " (2��) b+ c/2="1 " (2��)
��3��a+c="2 " (2��) b+ c/2="1 " (2��)
��

��ϰ��ϵ�д�
 Сѧѧϰ�ð���ϵ�д�
Сѧѧϰ�ð���ϵ�д� Сѧͬ�����������ܾ�ϵ�д�
Сѧͬ�����������ܾ�ϵ�д�
�����Ŀ
 cC(g)����ƽ�������������ϵ��ѹǿ(�¶Ȳ���)���±��г��˲�ͬѹǿ�·�Ӧ����ƽ��ʱ����A��Ũ�ȣ�
cC(g)����ƽ�������������ϵ��ѹǿ(�¶Ȳ���)���±��г��˲�ͬѹǿ�·�Ӧ����ƽ��ʱ����A��Ũ�ȣ� CO2��g����H2��g����K��1��850��ʱ�������ݻ�Ϊ2L���ܱ�������ͬʱ����1.0mol CO��3.0mol H2O��1.0mol CO2��xmol H2��
CO2��g����H2��g����K��1��850��ʱ�������ݻ�Ϊ2L���ܱ�������ͬʱ����1.0mol CO��3.0mol H2O��1.0mol CO2��xmol H2�� CH3OH(g) ��H < 0
CH3OH(g) ��H < 0 Ũ��Ϊ0.6mol/L��������¶��µĻ�ѧƽ�ⳣ����
Ũ��Ϊ0.6mol/L��������¶��µĻ�ѧƽ�ⳣ���� �����������ڴ��������е�
�����������ڴ��������е� �����������
����������� �Ļ�ѧ��ӦΪ��
�Ļ�ѧ��ӦΪ�� 2N2��g����3H2O��g��
2N2��g����3H2O��g��  H<0
H<0 O2(g)+ 2NO(g)����H��0���ﵽ
O2(g)+ 2NO(g)����H��0���ﵽ ƽ�⡣���ı�����һ������X��Y��X�ı仯����ͼ�����ߵ���
ƽ�⡣���ı�����һ������X��Y��X�ı仯����ͼ�����ߵ���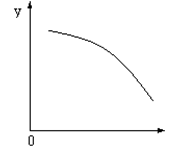
 pC(g)+qD (g) DH <0�������жϣ�������ȷ���� �� ��
pC(g)+qD (g) DH <0�������жϣ�������ȷ���� �� �� 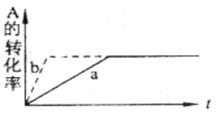
 �������1������ʱA��Ũ�ȱ�Ϊԭ����0��6������m+n<q+p
�������1������ʱA��Ũ�ȱ�Ϊԭ����0��6������m+n<q+p CH3OH(��)��6��ʱ��ϵ�ﵽƽ�⣬��ʱ��������������ʵ���Ϊ��ʼʱ��0.6������1��H2�ķ�Ӧ���� ��
CH3OH(��)��6��ʱ��ϵ�ﵽƽ�⣬��ʱ��������������ʵ���Ϊ��ʼʱ��0.6������1��H2�ķ�Ӧ���� �� ����һ��ʱ����Ӧ2NO(g)��O2(g)
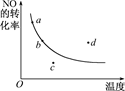
����һ��ʱ����Ӧ2NO(g)��O2(g)  2NO2(g) ��H<0��NO��ת�������¶ȱ仯��ϵ����ͼ��ͼ����a��b��c��d�ĸ��㣬���б�ʾδ�ﵽƽ��״̬����v��<v���ĵ���
2NO2(g) ��H<0��NO��ת�������¶ȱ仯��ϵ����ͼ��ͼ����a��b��c��d�ĸ��㣬���б�ʾδ�ﵽƽ��״̬����v��<v���ĵ���