��Ŀ����
��֪�ں�HNO3����Һ�з���Al������H2��ij��ɫ��Һ�ֻ��������11�������еļ��֣�Mg2����Fe3+��H����Ag����Ba2����SO42����HCO3����OH����MnO4����NO3����CO32������֪����Һ�ܸ���������Ӧ���ҷų�������ֻ���������Իش�
��1������Һ������Ӧֻ��AlO2�����ɣ���ԭ��Һһ�����еĴ�����������________(�ѧʽ)��������Ӧ�����ӷ���ʽ��_____________________________________________________�������ܺ��еĽ϶��������__________________(�ѧʽ)��
��2������Һ������Ӧ����Al3�����ɣ���ԭ��Һ�п��ܣ�����һ���ܣ��������ڵ�������_____________��
��1��Ba(OH)2 2Al��2OH����2H2O=2AlO2����3H2�� Ba(NO3)2 ��2��H+ SO42- Mg2+
�����������������Һ�ܸ���������Ӧ���ҷų�������ֻ����������˵������Һ���������ԣ�Ҳ�����Լ��ԣ�����Ϊ����Һ����ɫ�ģ�����һ��û��Fe3+��MnO4�����ݴ˿����жϡ�
��1������Һ������Ӧֻ��AlO2�����ɣ���˵����һ���Լ��ԣ���Mg2����H����Ag����HCO3��һ�����ܴ������ڡ�������Һ�ĵ����Կ�֪��һ������Ba2�������Ծ�һ��û��SO42����CO32�������ԭ��Һһ�����еĴ�����������Ba(OH)2����NO3������ȷ�������Ի����ܺ��еĽ϶��������Ba(NO3)2���йط�Ӧ�����ӷ���ʽΪ2Al��2OH����2H2O=2AlO2����3H2����
��2������Һ������Ӧ����Al3�����ɣ���˵������Һһ�������ԡ���HCO3����OH����CO32��һ�����ܴ������档����Ϊ�ں�HNO3����Һ�з���Al������H2�����Ը���Һ��Ҳһ�����ܴ�������NO3����������Һ�ĵ����Կ�֪����Һ��һ������SO42�������Ծ�һ�����ܴ�������Ag����Ba2��������ԭ��Һ�п��ܣ�����һ���ܣ��������ڵ�������)H+��SO42-��Mg2+��
���㣺�������ӹ����Լ����Ӽ�����й��ж�

�弰�仯����㷺Ӧ�����л��ϳɡ���ѧ����������
��1����ˮ�����������Ԫ�صı仯���£�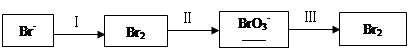
�ٹ��̢�ˮ�Լ��ԣ�����pH��3.5����ͨ��������
��.ͨ��������Ӧ�����ӷ���ʽ��______��
��.����ˮpH�����Cl2�������ʣ���ƽ��ԭ��������ԭ����______��
�ڹ��̢����ȿ�������ϳ�������Ũ̼������Һ���ա���ɲ���ƽ���з���ʽ��
Br2�� Na2CO3��
Na2CO3�� NaBrO3��
NaBrO3�� CO2��
CO2�� ______
______
�۹��̢��������ữ�ɵ�Br2��Na2SO4�Ļ����Һ��
��ͬ�����£����������ữ������������������٣�ԭ����______��
��2��NaBrO3��һ�ַ����Լ����������ữ��NaI��Һ����μ���NaBrO3��Һ��������2.6 mol NaBrO3ʱ����÷�Ӧ����Һ����͵�Ĵ�����ʽ�����ʵ����ֱ�Ϊ��
| ���� | I2 | Br2 | IO3- |
| ���ʵ���/mol | 0.5 | 1.3 | |
ijNa2CO3��NaAlO2�Ļ����Һ����μ���1 mol��L-1������,�����Һ�е�C ��HC
��HC ��Al
��Al ��Al3+�����ʵ�����������������仯��ϵ��ͼ��ʾ,������˵����ȷ���ǣ� ��
��Al3+�����ʵ�����������������仯��ϵ��ͼ��ʾ,������˵����ȷ���ǣ� ��
A��ԭ�����Һ�е�C ��Al ��Al �����ʵ���֮��Ϊ1��2 �����ʵ���֮��Ϊ1��2 |
| B��V1��V2=1��5 |
| C��M��ʱ���ɵ�CO2Ϊ0.05 mol |
D��a�߱�ʾ�����ӷ���ʽΪ:Al +H++H2O +H++H2O Al(OH)3�� Al(OH)3�� |
 ��Cu2+��Ba2+��Al3+��Ag+��Fe3+�������ӿ�����Cl-��
��Cu2+��Ba2+��Al3+��Ag+��Fe3+�������ӿ�����Cl-�� ����֪��
����֪��