��Ŀ����
18����ɫ�п�ѧ�Ҵ��ᰣ��•л���������֡����塱������2011��ŵ������ѧ���Լ��������ڣ��������ںܶ�Ӧ������չȭ�š������������첻ճ������������ܡ��ȵ�ת���豸�ȣ���1���������ڱ��е�λ�õ������ڢ��壬����ԭ�ӽṹʾ��ͼΪ
 ��
����2����ҵ�����õ�ԭ����Al2O3����Al2O3��AlCl3����������һ�ֵ�ԭ����AlCl3�ǹ��ۻ����������״̬�²����磻
��3��ͭ�ڳ�ʪ�Ŀ����л�����ͭ�̣�д���÷�Ӧ����ʽ2Cu+CO2+O2+H2O=Cu2��OH��2CO3��
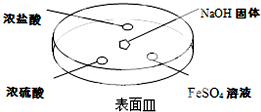
��4���õ�ⷨ��Fe��OH��2���������п����������Һ����BC
A������ˮ B��NaCl��Һ C��NaOH��Һ D��CuCl2��Һ��
���� ��1����Ԫ����26��Ԫ�أ�λ�����ڱ��еĵ������ڵڢ����壬��Ԫ��ԭ����13���˵�����������������Ӳ㣬�����3�����ӣ�
��2����ҵ���ǵ�����ڵ��������Ʊ����������Ȼ����ǹ��ۻ����
��3��ͭ�ڳ�ʪ�Ŀ����л�����ͭ����ͭ�Ϳ����е�ˮ������������������̼��Ӧ���ɼ�ʽ̼��ͭ��
��4����ⷨ�Ʊ�������������������������Һ���������������õ����ӷ�����ԭ��Ӧ������������Ũ������Ӧ����������������
��� �⣺��1����Ԫ����26��Ԫ�أ�λ�����ڱ��еĵ������ڵڢ����壬��Ԫ��ԭ����13���˵�����������������Ӳ㣬�����3�����ӣ�ԭ�ӽṹʾ��ͼΪ�� ���ʴ�Ϊ���������ڢ��壻
���ʴ�Ϊ���������ڢ��壻 ��
��
��2����ҵ���ǵ�����ڵ��������Ʊ����������Ȼ����ǹ��ۻ�����������״̬�²����磻
�ʴ�Ϊ��Al2O3��AlCl3�ǹ��ۻ����������״̬�²����磻
��3��ͭ�ڳ�ʪ�Ŀ����л�����ͭ����ͭ�Ϳ����е�ˮ������������������̼��Ӧ���ɼ�ʽ̼��ͭ����Ӧ�Ļ�ѧ����ʽΪ��2Cu+CO2+O2+H2O=Cu2��OH��2CO3 ��
�ʴ�Ϊ��2Cu+CO2+O2+H2O=Cu2��OH��2CO3 ��
��4����ⷨ�Ʊ�������������������������Һ���������������õ����ӷ�����ԭ��Ӧ������������Ũ������Ӧ����������������
A����ˮ�����Բ���������Ʊ���������������A����
B������Ȼ�����Һ�����缫��Ӧ��Fe-2e-=Fe2+������2H++2e-=H2����ˮ���뱻�ƻ�������У�����������Ũ�������������������������������������B��ȷ��
C���������������Һ�缫��Ӧ��Fe-2e-=Fe2+������2H++2e-=H2�������ɵ��������ӽ����������������������������������C��ȷ��
D������Ȼ�ͭ��Һ���缫��Ӧ��Fe-2e-=Fe2+������Cu2++2e-=Cu�������Ʊ���������������D����
�ʴ�Ϊ��BC��
���� ���⿼���������Ʊ��ķ�����ԭ�����������ԭ��ʵ������Ӧ�ã����ջ����ǽ���ؼ�����Ŀ�Ѷ��еȣ�

 �¿α�����Ķ�ѵ��ϵ�д�
�¿α�����Ķ�ѵ��ϵ�д� ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д�| A�� | Ϊ����ǿ���������Һ��������������ϡ���Ὣ���������Һ�����ữ | |
| B�� | ���Ʊ�Fe��OH��3���壬��ʢ�з�ˮ���ձ��еμ�FeCl3������Һ����ʱ����� | |
| C�� | ��ϡ����ϴ��ʢ�Ź�ʯ��ˮ���Լ�ƿ | |
| D�� | ��������������Ϊ10%��ZnSO4��Һ����10 g ZnSO4•7H2O�ܽ���90 gˮ�� |
| A�� | ������ˮ�ĵ����һ����ǿ����ʣ�������ˮ�ĵ����һ����������� | |
| B�� | ǿ�������Һ�ĵ�������һ�������������Һǿ | |
| C�� | NaCl��Һ�ڵ����������µ���������Ӻ������� | |
| D�� | �Ȼ��ƾ��岻�����������Ȼ��ƾ����в����������ƶ������� |
| A�� | 2Fe3++SO2+2H2O�T2Fe2++SO42-+4H+ | B�� | 2Fe3++H2S�T2Fe2++S��+2H+ | ||
| C�� | I2+SO2+2H2O�T2I-+SO42-+4H+ | D�� | 2Br-+SO42-+4H+�TBr2+SO2��+2H2O |
 ����NH3���ȶ��Ա�PH3ǿ����д��ǿ������������
����NH3���ȶ��Ա�PH3ǿ����д��ǿ������������