��Ŀ����
���ǵ����Ϻ����ḻ��һ��Ԫ�أ������仯�����ڹ�ũҵ������������������Ҫ���á���ش��������⣺
��1����ͼ��N2��H2��Ӧ����2 mol NH3������������
��ʾ��ͼ��д������NH3���Ȼ�ѧ����ʽ��
_____________________________________________
___________________________��
��2������̬��̬ԭ���γ�1 mol��ѧ���ͷŵ���������м��ܡ��ӻ�ѧ���ĽǶȷ�������
ѧ��Ӧ�Ĺ��̾��Ƿ�Ӧ��Ļ�ѧ�����ƻ���������Ļ�ѧ�����γɹ��̡��ڻ�ѧ��Ӧ��
���У���ѧ����Ҫ�����������γɻ�ѧ���ֻ��ͷ�������
��֪��ӦN2(g)��3H2(g)??2NH3(g)����H��a kJ��mol��1��
�Ը��ݱ������м������ݹ���a����ֵ��________��
| ��ѧ�� | H��H | N��H | N��N |
| ����kJ��mol��1 | 436 | 391 | 945 |
��1��N2(g)��3H2(g)??2NH3(g) ��H����92.2 kJ��mol��1����2����93
���������������1������������ϵͼ���÷�Ӧ�Ħ�H��E1��E2��(335��427.2)kJ��mol��1����92.2 kJ��mol��1����2����H�����յ��������ͷŵ�������(945��3��436��6��391)kJ��mol��1����93 kJ��mol��1��
���㣺���⿼����ͨ�����ܴ�С�����㷴Ӧ�ȡ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д���֪H-H��Cl-Cl��H-Cl�ļ��ֱܷ�Ϊ436 kJ��mol-1��243 kJ��mol-1��431 kJ��mol-1�����ô����ݹ��ƣ���Cl2��H2����2mol H-Cl��ʱ����ЧӦ��H���ڣ� ��
| A��-183 kJ��mol-1 | B��-91��5kJ��mol-1���� |
| C��+183kJ��mol-1������������������������ | D��+ 91��5kJ��mol-1 |
�������������Ȼ�ѧ����ʽ��
H2��g��+ O2��g��=H2O��g������H=" a" kJ/mol
O2��g��=H2O��g������H=" a" kJ/mol
H2��g��+ O2��g��=H2O��l������H=" b" kJ/mol
O2��g��=H2O��l������H=" b" kJ/mol
2H2��g��+ O2��g��=2H2O��l������H=" c" kJ/mol
�������ǵ����б�������ȷ����
| A�����Ƕ������ȷ�Ӧ | B��a��b��c��Ϊ��ֵ |
| C����Ӧ�ȵĹ�ϵ��a=b | D����Ӧ�ȵĹ�ϵ��2b=c |
��֪�����Ȼ�ѧ����ʽ��
Zn(s)��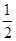 O2(g)=ZnO(s)����H1����351.1 kJ��mol��1
O2(g)=ZnO(s)����H1����351.1 kJ��mol��1
Hg(l)��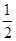 O2(g)=HgO(s)����H2����90.7 kJ��mol��1
O2(g)=HgO(s)����H2����90.7 kJ��mol��1
�ɴ˿�֪Zn(s)��HgO(s)=ZnO(s)��Hg(l)����H3�����Ц�H3��ֵ�� (����)
| A����441.8 kJ��mol��1 | B����254.6 kJ��mol��1 |
| C����438.9 kJ��mol��1 | D����260.4 kJ��mol��1 |
��֪��H+(aq) + OH��(aq)��H2O(l) ��H����57.3kJ/mol����1L 0.5mol/L��NaOH��Һ�м����������ʣ���ϡ�����ŨH2SO4����ϡ���ᣬǡ����ȫ��Ӧ����ЧӦ��H1����H2����H3�Ĺ�ϵ��ȷ����
| A����H1����H2����H3 | B����H3����H1����H2 |
| C����H1����H3����H2 | D����H2����H1����H3 |
��֪��
��1��Fe2O3(s) �� C(s)=
C(s)= CO2(g)��2Fe(s) ��H����234��1 kJ/mol
CO2(g)��2Fe(s) ��H����234��1 kJ/mol
��2��C(s)��O2(g)=CO2(g) ��H����393��5 kJ/mol
��2Fe(s)�� O2(g)=Fe2O3(s) �Ħ�H�ǣ� ��
O2(g)=Fe2O3(s) �Ħ�H�ǣ� ��
| A����824��4 kJ/mol | B����627��6 kJ/mol |
| C����744��7 kJ/mol | D����169��4 kJ/mol |
S(��б)��S(����)���������ͬ�������塣
��֪����S(��б��s)��O2(g)=SO2(g) ��H1����297.16 kJ��mol��1
��S(������s)��O2(g)=SO2(g) ��H2����296.83 kJ��mol��1
��S(���s)=S(������s) ��H3
����˵����ȷ���ǣ� ��
| A����H3����0.33 kJ��mol��1 |
| B����б��ת��Ϊ������ķ�Ӧ�����ȷ�Ӧ |
| C��S(��б��s)=S(������s) ��H3<0��������ȵ�б���ȶ� |
| D��S(��б��s)=S(������s) ��H3>0����б����������ȶ� |
��ҵ����ˮú���ķ�ӦΪ��C(s)��H2O(g)=CO(g)��H2(g) ��H����131.4 kJ/mol�������ж���ȷ���ǣ� ��
| A����Ӧ�������ܺ�С�������������ܺ� |
| B��CO(g)��H2(g)=C(s)��H2O(g) ��H����131.4 kJ/mol |
| C��ˮú����Ӧ�У�����1 mol H2(g)����131.4 kJ���� |
| D��ˮú����Ӧ������1���CO(g)����131.4 kJ���� |
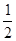 O2(g)�TCO(g) �� ��H2
O2(g)�TCO(g) �� ��H2