��Ŀ����
��a��b��c��d����ԭ��������������ij���������Ԫ�أ�a��c��Ϲ��ɻ�����x��b��c��Ϲ��ɻ�����y��c��d��Ϲ��ɻ�����z��Ԫ����ɵĵ��ʣ�����a��b��c��d��ʾ������ɵĻ�����֮��ķ�Ӧ��ϵ���£�δ��ƽ����
��x+z��c��+n������y+z��c��+m������n+y��m+x������d+x��n+a��
��1��������Ӧ�У��г������˵���÷�Ӧһ������������ԭ��Ӧ������������
���Ӧ��ţ���
��2����ɻ�����n��Ԫ��Ϊ����������������ĸ�����ж�����Ϊ������������������
��3����������д����z�ĵ���ʽΪ������������y�ĽṹʽΪ����������������
��4����������д������Ӧ�ٵĻ�ѧ����ʽ������������������������������������
��x+z��c��+n������y+z��c��+m������n+y��m+x������d+x��n+a��
��1��������Ӧ�У��г������˵���÷�Ӧһ������������ԭ��Ӧ������������
���Ӧ��ţ���
��2����ɻ�����n��Ԫ��Ϊ����������������ĸ�����ж�����Ϊ������������������
��3����������д����z�ĵ���ʽΪ������������y�ĽṹʽΪ����������������
��4����������д������Ӧ�ٵĻ�ѧ����ʽ������������������������������������
����8�֣�
��1���٢ڢܣ�1�֣�
��2��acd��1�֣�
���ݷ�Ӧ�ٿ���ȷ��nһ������a��d����Ԫ�أ����ݷ�Ӧ�ܿ���ȷ��nһ������c��d����Ԫ�أ�����nһ������a��c��d����Ԫ�أ�2�֣�
��3��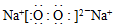 ��1�֣�����O��C��O��1�֣�
��1�֣�����O��C��O��1�֣�
��4��2Na2O2+2H2O 4NaOH+O2����2�֣�
4NaOH+O2����2�֣�
��1���٢ڢܣ�1�֣�
��2��acd��1�֣�
���ݷ�Ӧ�ٿ���ȷ��nһ������a��d����Ԫ�أ����ݷ�Ӧ�ܿ���ȷ��nһ������c��d����Ԫ�أ�����nһ������a��c��d����Ԫ�أ�2�֣�
��3��
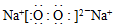 ��1�֣�����O��C��O��1�֣�
��1�֣�����O��C��O��1�֣���4��2Na2O2+2H2O
 4NaOH+O2����2�֣�
4NaOH+O2����2�֣������ͻ�ƿ��ڢ�x + z��c�� + n������y + z��c�� + m��������ѧ��Ӧ����ʽ������ѧ��ѧ�У�������z�뻯����x��������y��Ӧ�������嵥�����ɵģ����ϵ���Na2O2��CO2��H2O��Ӧ��������O2�ķ���ʽ���ɴ˷����ɵã�a��b��c��d����Ԫ�طֱ�Ϊ��H��C��O��Na����Ԫ�ء�

��ϰ��ϵ�д�
�����Ŀ
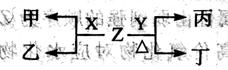
 Cn
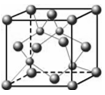
Cn Cn��Ϊͬλ�أ�����������֮��Ϊ8
Cn��Ϊͬλ�أ�����������֮��Ϊ8
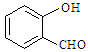 ���Ͷ��ǻ�����ȩ��
���Ͷ��ǻ�����ȩ��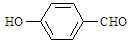 ����ǰ�߷е���ں��ߣ�������
����ǰ�߷е���ں��ߣ�������