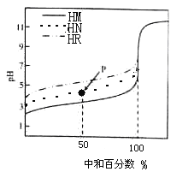
��Ŀ����
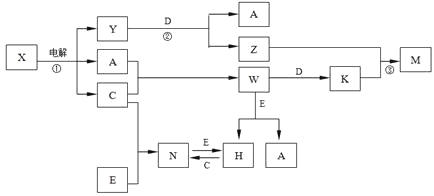
����Ŀ��A��B��C��D��E����ѧ��ѧ�������ʣ� X��Y��Z��M��N��W��H��K�dz��������X��B��C�Ļ��ϲ������֮��������ת����ϵ����Ӧ��Ͳ����е�H2O����ȥ����
��1��Y�ĵ���ʽΪ ������E���ʵ�Ԫ�������ڱ���λ��______________��
��2����Ӧ�������ӷ���ʽΪ ��Ӧ�������ӷ���ʽΪ
��3��ij������C��Ư�ۡ�
��д����Ư�۵Ļ�ѧ����ʽ
��Ϊ�ⶨ�ù����Ƶõ�Ư������Ч�ɷֵĺ�����ijС�����������ʵ�飺��ȡƯ��2.0g����ĥ���ܽ⣬���Ƴ�250mL��Һ��ȡ��25.00mL���뵽��ƿ�У��ټ��������KI��Һ���������ᣬ��ʱ���������ӷ���ʽΪ�� �����á�����ȫ��Ӧ����0.1mol��L-1��Na2S2O3��Һ������Һ�ζ���Ӧ���ɵĵ⣬��֪��ӦʽΪ��2Na2S2O3+I2=Na2S4O6+2NaI������ȥNa2S2O3��Һ20.00mL�����Ư������Ч�ɷֵ���������Ϊ ��������С�������λ����
���𰸡���1��![]() ��4��������
��4��������
��2��2Cl-+2H2O![]() 2OH��+Cl2��+H2����Al3++3AlO2��+6H2O=4Al(OH)3��
2OH��+Cl2��+H2����Al3++3AlO2��+6H2O=4Al(OH)3��
��3����2Cl2+2Ca(OH)2=CaCl2+Ca(ClO)2+2H2O
��ClO��+2I��+2H+=I2+Cl��+H2O��35.75%
�����������������A��B��C��D��E����ѧ��ѧ�������ʣ��������е�ת����ϵ��X![]() A+C+Y�����Գ����ƶ�XΪNaCl��Һ���������H2��Cl2��NaOH��A��B��C��D��E����ѧ��ѧ�������ʣ�X��B��C�Ļ��ϲ������BΪNa��CΪCl2������Yһ����NaOH��Y+D��A+Z���˷�Ӧ��D���ʺ�����������Һ��Ӧ����A���ʣ�ͨ����ѧDΪ��������AΪH2��ZΪNaAlO2��CΪCl2��A+C��W��WΪHCl��W+D��K��KΪAlCl3��Z+K��M��MΪAl��OH��3����֤3��a+b��=2��a+c��=3��d-a���������ƶϷ��ϣ�����ת����ϵ��E�ǵ��ʣ�W��HCl��+E��H+A��H2����H+C��Cl2����N��N+E��H��˵��E�DZ��Ԫ�ص��ʣ����ᷴӦ֤���ǽ������ʣ������ж�EΪFe��H��FeCl2����NΪ��FeCl3�����ת����ϵ�������⣻��������ת����ϵ�е����ʷֱ��ǣ�A��H2��B��Na��C��Cl2��D��Al��E��Fe��H��FeCl2��K��AlCl3��M��Al��OH��3��N��FeCl3��W��HCl��X��NaCl��Y��NaOH��Z��NaAl02��
A+C+Y�����Գ����ƶ�XΪNaCl��Һ���������H2��Cl2��NaOH��A��B��C��D��E����ѧ��ѧ�������ʣ�X��B��C�Ļ��ϲ������BΪNa��CΪCl2������Yһ����NaOH��Y+D��A+Z���˷�Ӧ��D���ʺ�����������Һ��Ӧ����A���ʣ�ͨ����ѧDΪ��������AΪH2��ZΪNaAlO2��CΪCl2��A+C��W��WΪHCl��W+D��K��KΪAlCl3��Z+K��M��MΪAl��OH��3����֤3��a+b��=2��a+c��=3��d-a���������ƶϷ��ϣ�����ת����ϵ��E�ǵ��ʣ�W��HCl��+E��H+A��H2����H+C��Cl2����N��N+E��H��˵��E�DZ��Ԫ�ص��ʣ����ᷴӦ֤���ǽ������ʣ������ж�EΪFe��H��FeCl2����NΪ��FeCl3�����ת����ϵ�������⣻��������ת����ϵ�е����ʷֱ��ǣ�A��H2��B��Na��C��Cl2��D��Al��E��Fe��H��FeCl2��K��AlCl3��M��Al��OH��3��N��FeCl3��W��HCl��X��NaCl��Y��NaOH��Z��NaAl02��
��1��YΪNaOH�������ӻ������д�ĵ���ʽΪ��![]() ���E���ʵ�Ԫ����������Ԫ��λ�ڵ�4�������壻
���E���ʵ�Ԫ����������Ԫ��λ�ڵ�4�������壻
��2����2����Ӧ���ǵ�ⱥ��ʳ��ˮ�����ӷ���ʽΪ2Cl-+2 H2O![]() 2OH��+Cl2��+ H2�� ��ת����ϵ�з�Ӧ����Z��NaAl02��+K��AlCl3����M��Al��OH��3�������ӷ���ʽΪ��Al3++3AlO2-+6H2O=4Al��OH��3����
2OH��+Cl2��+ H2�� ��ת����ϵ�з�Ӧ����Z��NaAl02��+K��AlCl3����M��Al��OH��3�������ӷ���ʽΪ��Al3++3AlO2-+6H2O=4Al��OH��3����
��3����д����Ư�۵Ļ�ѧ����ʽ2Cl2+2Ca(OH)2=CaCl2+Ca(ClO)2+2H2O��
��Ư����Һ�ټ��������KI��Һ���������ᣬ��ʱ���������ӷ���ʽΪ��ClO��+2I��+2H+=I2+Cl��+H2O��
��2Na2S2O3+I2=Na2S4O6+2NaI����ϵʽCa��ClO��2��2Cl2��2I2��4Na2S2O3��
n[Ca��ClO��2]=![]() n��Na2S2O3��="20.0" mL��10-3L mL-1��0.1 mol L-1��
n��Na2S2O3��="20.0" mL��10-3L mL-1��0.1 mol L-1��![]() ="0.005" mol��
="0.005" mol��
Ca��ClO��2%=![]() ��100%=35.75%��
��100%=35.75%��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ����ȥ�����������������ʣ�������Ϊ���ʣ�����ѡ���Լ���������������ȷ��һ���ǣ� ��
ѡ�� | ���� | ѡ�õ��Լ� | �������� |
�� | CaO��CaCO3�� | ˮ | �ܽ⡢���ˡ��ᾧ |
�� | CO2��CO�� | ���� | ��ȼ |
�� | CuSO4��H2SO4�� | ����������Һ | ���� |
�� | Cu��CuO�� | ϡ���� | �ܽ⡢���ˡ�ϴ�ӡ����� |
A���� B���� C���� D����