��Ŀ����
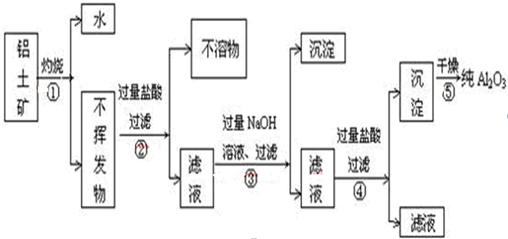
��10�֣�����������Ҫ�ɷ���Al2O3����RO2��Fe2O3�����ʣ�����ȡAl2O3��һ�ֹ���������ͼ13��ʾ��
��1��Ԫ��Rλ�����ڱ��еĵ������ڣ�����������ϼۺ����ϼ۵ľ���ֵ��ȡ�RO2����;�У�д��1�����ɣ� ��
��2����������������ռ���Һ����Ҫ��Ӧ�����ӷ���ʽΪ
�� ��
�� ��
��3����ҵ��ͨ������������ͱ���ʯ������Һ��ұ������������֪�缫����Ϊ���Բ��ϣ�������ӦʽΪ ��
��4���������뽹̿�Ļ�����ڵ����и��¼��ȷ�Ӧ���Ƶ����ͷǽ�������AIN��һ����ѧ��������X����֪ÿת��3 mol e��,��1.5mol������X���ɣ��˷�Ӧ�Ļ�ѧ����ʽ ��
��1���������ˡ���Ʒ���������ϡ�ʯӢ������ʯӢ������ ������������������1��
��2����Al2O3+2OH�� = 2AlO2��+H2O ����������������������������������2��
��SiO2+2OH��= SiO32��+ H2O ����������������������������������2��
��3��2O2�� - 4 e�� = O2�� ������������������������������������������2��
��4��Al2O3+N2+3C 2AlN+3CO ����������������������������3��
����ѧ����ʽ��д����Ӧ���������塢��������1�֣���ƽ1�֣���ѧʽ��ṹ��ʽд��1����1�֡�����ͬ��
����:

 �����ҵ���������ϵ�д�
�����ҵ���������ϵ�д� �����̸�Ӯ����ٸ�Ч�����ܸ�ϰ���ϿƼ�������ϵ�д�
�����̸�Ӯ����ٸ�Ч�����ܸ�ϰ���ϿƼ�������ϵ�д� �����ҵ�����������ѧ���ӳ�����ϵ�д�
�����ҵ�����������ѧ���ӳ�����ϵ�д� ����ѧ��Ӯ�����ϵ�д�
����ѧ��Ӯ�����ϵ�д�