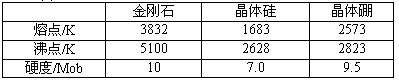
��Ŀ����
(15��)X��Y��Z���ֶ�����Ԫ�أ�����֮��Ļ�������XY2��Z2Y��XY3��Z2Y2��Z2X�ȡ���֪Ym-��Zn+�������Ӿ�����ͬ�ĵ��Ӳ�ṹ��Xm-��Ym-��һ����ӡ��Իش�
(1)Xԭ�ӵ�ԭ�ӽṹʾ��ͼ��__________�����������ڱ���___________����__________�塣
(2)Z2Y2�ĵ���ʽΪ___________��Z2Y�ĵ���ʽΪ___________��
(3)XY3�ڱ�״��������״���壬������___________���塣��֪XY3��ˮ������Z2Y2����Ӧ������һ�־���Ư�����õ�ǿ������H2Y2���ƲⷴӦʱ�Ļ�ѧ����ʽΪ___________��
(4)Z2X����___________���壻Z2X��ˮ��Һ��XY3��ˮ��������Һ�з�Ӧ�����ӷ���ʽ��
______________________________________________________________________________��
(5)��X��Y��Z��������Ԫ������ɵ����ֻ�������Һ����Ӧ������������ӷ���ʽ��
_______________________________________________________________________________
(1)Xԭ�ӵ�ԭ�ӽṹʾ��ͼ��__________�����������ڱ���___________����__________�塣
(2)Z2Y2�ĵ���ʽΪ___________��Z2Y�ĵ���ʽΪ___________��
(3)XY3�ڱ�״��������״���壬������___________���塣��֪XY3��ˮ������Z2Y2����Ӧ������һ�־���Ư�����õ�ǿ������H2Y2���ƲⷴӦʱ�Ļ�ѧ����ʽΪ___________��
(4)Z2X����___________���壻Z2X��ˮ��Һ��XY3��ˮ��������Һ�з�Ӧ�����ӷ���ʽ��
______________________________________________________________________________��
(5)��X��Y��Z��������Ԫ������ɵ����ֻ�������Һ����Ӧ������������ӷ���ʽ��
_______________________________________________________________________________
(1)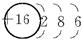 ���� ��A
���� ��A
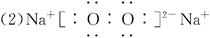
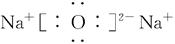
(3)���� H2SO4+Na2O2====Na2SO4+H2O2
(4)���� 2H++S2-====H2S��
(5)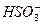 +H+====SO2��+H2O
+H+====SO2��+H2O
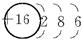 ���� ��A
���� ��A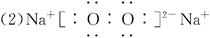
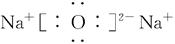
(3)���� H2SO4+Na2O2====Na2SO4+H2O2
(4)���� 2H++S2-====H2S��
(5)
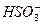 +H+====SO2��+H2O
+H+====SO2��+H2OXm-��Ym-������ͬ��ɣ���֪X��Yλ��ͬһ���壬�����γ�XY2��XY3�ͻ������Z2Y��֪YӦ��-2�ۡ��ɴ˿��Ƶ�XΪSԪ�أ�YΪOԪ�ء���ѧ����ʽΪ��NaHSO4+NaHSO4====Na2SO4+SO2��+H2O

��ϰ��ϵ�д�
�����Ŀ
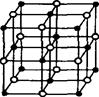
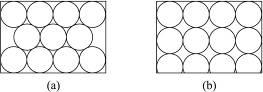
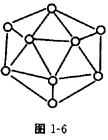
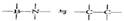 ���ȵ��������ʣ���ṹ�����ʾ��м���������ԣ������֪����BN�����־����У�һ��������_________�Ŀռ���״�ṹ���壬��������ĥ���ϣ���һ����������_________�IJ�״�ṹ�Ļ���;��壬���������ϣ�����ṹ��ÿһ������С�ķ�ջ����� _________��Bԭ�ӣ�B��N������Ϊ_________��
���ȵ��������ʣ���ṹ�����ʾ��м���������ԣ������֪����BN�����־����У�һ��������_________�Ŀռ���״�ṹ���壬��������ĥ���ϣ���һ����������_________�IJ�״�ṹ�Ļ���;��壬���������ϣ�����ṹ��ÿһ������С�ķ�ջ����� _________��Bԭ�ӣ�B��N������Ϊ_________��