��Ŀ����
����Ŀ����֪X��Y��Z����Ԫ�ص�ԭ������֮�͵���42��XԪ��ԭ�ӵ�4p�������3��δ�ɶԵ��ӣ�YԪ��ԭ�ӵ������2p�������2��δ�ɶԵ��ӡ�X��Y���γɻ�����X2Y3��ZԪ�ؿ����γɸ�һ�����ӡ�
��ش��������⣺
��1��XԪ��ԭ�ӻ�̬ʱ�ĵ����Ų�ʽΪ______________����Ԫ�صķ�����________��
��2��YԪ��ԭ�ӵļ۲���ӵĵ����Ų�ͼΪ____________________����Ԫ�ص�������________��
��3����֪������X2Y3��ϡ������Һ�пɱ�����п��ԭΪXZ3�����ﻹ��ZnSO4��H2O���÷�Ӧ�Ļ�ѧ����ʽ��_________________________________________________��
��4���Ƚ�X���⻯����ͬ��ڶ�����������Ԫ�����γɵ��⻯���ȶ��ԣ���˵������_____________________________________________________________________��
���𰸡���1��1s22s22p63s23p63d104s24p3��As��
��2��![]() ������
������
��3��As2O3+6Zn+6H2SO4�T2AsH3��+6ZnSO4+3H2O��
��4���ȶ��ԣ�NH3��PH3��AsH3����Ϊ����Խ�̣�����Խ������Խ�ȶ���
�����������������XԪ��ԭ�ӵ�4p�������3��δ�ɶԵ��ӣ������֪��XԪ��ԭ�ӻ�̬ʱ�ĵ����Ų�ʽΪ1s22s22p63s23p63d104s24p3��ΪAs��YԪ��ԭ�ӵ������2p�������2��δ�ɶԵ��ӣ���Ԫ��ԭ�ӻ�̬ʱ�ĵ����Ų�ʽΪ1s22s22p4��ΪO��X��Y��Z����Ԫ�ص�ԭ������֮�͵���42��Xԭ������Ϊ33��YΪ8����ZΪHԭ�ӣ�X��Z���γɻ�����AsH3���û�����Ŀռ乹�ͺͰ������ơ�
��1�������Ϸ�����֪XΪAs�������Ų�ʽΪ1s22s22p63s23p63d104s24p3���ʴ�Ϊ��1s22s22p63s23p63d104s24p3��As��
��2��YΪ��Ԫ�أ�ԭ�ӵļ۲���ӵĹ����ʾʽΪ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ������
������
��3����֪������As2O3��ϡ������Һ�пɱ�����п��ԭΪAsH3�����ﻹ��ZnSO4��H2O����Ӧ����ʽΪ��As2O3+6Zn+6H2SO4�T2AsH3��+6ZnSO4+3H2O���ʴ�Ϊ��As2O3+6Zn+6H2SO4�T2AsH3��+6ZnSO4+3H2O��
��4��XΪNԪ�أ��ǽ����Խ�ǿ����Ϊ����Խ�̣�����Խ������Խ�ȶ������ȶ��ԣ�NH3��PH3��AsH3���ʴ�Ϊ���ȶ��ԣ�NH3��PH3��AsH3����Ϊ����Խ�̣�����Խ������Խ�ȶ���

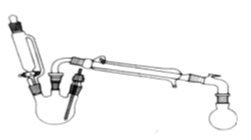
����Ŀ��ij�о���С��̽�����������ķ�Ӧ������ʵ�����£�
CH3COOH + C2H5OH ![]() CH3COOC2H5 + H2O
CH3COOC2H5 + H2O
Ͷ�� 1 �� 1 CH3COOHת���� 65%
1 �� 10 CH3COOHת���� 97%
(��120 ���²ⶨ)
��֪������������ʣ����³�ѹ��
�ܶ�g/mL | �۵�/�� | �е�/�� | ˮ���� | |
�Ҵ� | 0.79 | -114 | 78 | �� |
���� | 1.049 | 16.2 | 117 | �� |
�������� | 0.902 | 84 | 76.5 | ���� |
�ϳɷ�Ӧ��
������ƿ�м����Ҵ�5 mL������5 mL��2СƬ���Ƭ��©����������14.3 mL ���Ҵ�20 mL����������ͨ����ȴˮ��ʼ�������ȣ����Ƶμ��ٶȵ��������ٶȣ���Ӧ�¶Ȳ�����120 �档
�����ᴿ��
����Ӧ�ֲ��ﵹ���Һ©���У��������������͵�Na2CO3��Һ������NaCl��Һ������CaCl2��Һϴ�ӣ�����������ˮ̼��أ�����һ��ʱ�����ȥ̼��ء�����ͨ������õ�����������������
�ش��������⣺
��1��������Ӧ�Ļ���
���Ҵ��ǻ���ʾ��
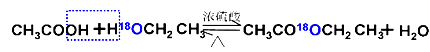
�ô����ǻ���ʾ��

����18ˮռ����ˮ����һ�룬��Ҳһ�������ʵ���Ʒ���������ӦΪ��ȡ����Ӧ����������������Ӧ�Ļ��� ��
2��������Ӧ��һ������ķ�Ӧ��120 ��ʱ��ƽ�ⳣ��K= ��
��3���������һ��ʱ��������ǼӴ�Ƭ��Ӧ�ò�ȡ����ȷ������________(����ȷ�𰸱��)��
A���������� B����ȴ�� C�����貹�� D����������
��4��Ũ�������Ҵ���λ�ϣ� ��
��5�����Ƶμ�������Ҵ����Һ���ٶȵ��������ٶ�Ŀ���ǣ� ��
��6�������Ĵ�������������Ҫ����Щ���ʣ� �����͵�Na2CO3��Һϴ�ӳ�ȥ���ᡣ����ж��Ƿ������ ���ñ���NaCl��Һϴ�ӳ�ȥ������Na2CO3��Һ��Ϊʲô����ˮ�� ��