��Ŀ����
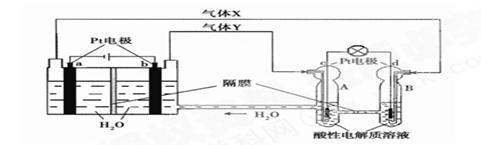
�������ȣ�ClO2����һ�ֻ���ɫ�д̼�����ζ�����壬���۵�Ϊ��59�棬�е�Ϊ11.0�棬������ˮ����ҵ�����Գ�ʪ��KClO3�Ͳ��ᣨH2C2O4����60��ʱ��Ӧ�Ƶá�ijѧ��������ͼ��ʾװ��ģ�ҵ��ȡ���ռ�ClO2��
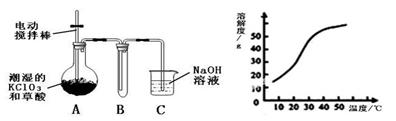
��1��A���������¶ȿ���װ�ã����ƾ��ơ��¶ȼ��⣬����Ҫ�IJ��������� ��
��2����Ӧ����װ��C�пɵ�NaClO2��Һ����֪���¶ȵ���38��ʱNaClO2������Һ������������NaClO2��3H2O�����¶ȸ���38��ʱ����������NaClO2����������ͼ��ʾ��NaClO2���ܽ�����ߣ��벹���NaClO2��Һ���Ƶ�NaClO2����IJ������裺 �� �����ᾧ���� ���� ϴ�ӣ��� ���
��3��ClO2�ܲ��ȶ������������ƣ���ˮ���յõ�ClO2��Һ��Ϊ�ⶨ������Һ��ClO2��Ũ�ȣ�����������ʵ�飺�� ȷ��ȡClO2��ҺV1mL���뵽��ƿ�У�����������ˮϡ�ͣ�����������pH��2.0���� ����������KI���壬����Ƭ�̡���ʱ������Ӧ�����ӷ���ʽΪ�� ���� �������ָʾ������c mol/L Na2S2O3��Һ�ζ������յ�ʱ����Na2S2O3��ҺV2 mL����ԭClO2��Һ��Ũ��Ϊ mol��L���ú���ĸ�Ĵ���ʽ��ʾ��������֪2 Na2S2O3+I2= Na2S4O6+2NaI��
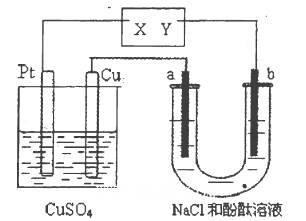
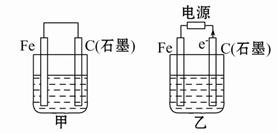
����Na+��Ba2+��Cu2+��SO42����Cl�� ����γɵ�����ǿ�������Һ���ֱ�װ����ͼװ��
�еļס��ҡ��������ձ��н��е�⣬�缫��Ϊʯī�缫��
��ͨ��Դ������һ��ʱ��������c�缫�������ӡ������¸��ձ�����ҺpH����ʱ��t�Ĺ�ϵ������ͼ�������������ܽ������Ӱ�죩���ݴ˻ش��������⣺
��1��д�����ձ��з�����Ӧ�Ļ�ѧ����ʽ ��
��2���缫f�Ϸ����ĵ缫��ӦΪ ��
��3��������һ��ʱ�������ձ���c�缫����������8g��Ҫʹ���ձ�����Һ�ָ���ԭ����״̬��Ӧ���еIJ����� ��

��1��A���������¶ȿ���װ�ã����ƾ��ơ��¶ȼ��⣬����Ҫ�IJ��������� ��
��2����Ӧ����װ��C�пɵ�NaClO2��Һ����֪���¶ȵ���38��ʱNaClO2������Һ������������NaClO2��3H2O�����¶ȸ���38��ʱ����������NaClO2����������ͼ��ʾ��NaClO2���ܽ�����ߣ��벹���NaClO2��Һ���Ƶ�NaClO2����IJ������裺 �� �����ᾧ���� ���� ϴ�ӣ��� ���
��3��ClO2�ܲ��ȶ������������ƣ���ˮ���յõ�ClO2��Һ��Ϊ�ⶨ������Һ��ClO2��Ũ�ȣ�����������ʵ�飺�� ȷ��ȡClO2��ҺV1mL���뵽��ƿ�У�����������ˮϡ�ͣ�����������pH��2.0���� ����������KI���壬����Ƭ�̡���ʱ������Ӧ�����ӷ���ʽΪ�� ���� �������ָʾ������c mol/L Na2S2O3��Һ�ζ������յ�ʱ����Na2S2O3��ҺV2 mL����ԭClO2��Һ��Ũ��Ϊ mol��L���ú���ĸ�Ĵ���ʽ��ʾ��������֪2 Na2S2O3+I2= Na2S4O6+2NaI��
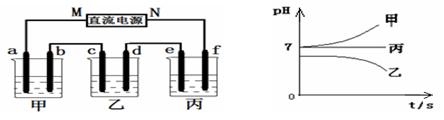
����Na+��Ba2+��Cu2+��SO42����Cl�� ����γɵ�����ǿ�������Һ���ֱ�װ����ͼװ��
�еļס��ҡ��������ձ��н��е�⣬�缫��Ϊʯī�缫��

��ͨ��Դ������һ��ʱ��������c�缫�������ӡ������¸��ձ�����ҺpH����ʱ��t�Ĺ�ϵ������ͼ�������������ܽ������Ӱ�죩���ݴ˻ش��������⣺
��1��д�����ձ��з�����Ӧ�Ļ�ѧ����ʽ ��
��2���缫f�Ϸ����ĵ缫��ӦΪ ��
��3��������һ��ʱ�������ձ���c�缫����������8g��Ҫʹ���ձ�����Һ�ָ���ԭ����״̬��Ӧ���еIJ����� ��
I�� (1) �ձ� (2��) ��2�� ���ȹ��� ��2 �֣�
��3��2ClO2 + 8H+ + 10I��="==2" Cl��+ 5I2 + 4H2O ��3 �֣�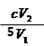 ��2 �֣�
��2 �֣�
��1��2CuSO4 + 2H2O 2Cu + O2�� + 2H2SO4��2�֣�
2Cu + O2�� + 2H2SO4��2�֣�
��2��4OH����4e��=2H2O + O2����2�֣�
��3������ձ��м���2.25gˮ��3�֣�
��3��2ClO2 + 8H+ + 10I��="==2" Cl��+ 5I2 + 4H2O ��3 �֣�
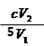 ��2 �֣�
��2 �֣���1��2CuSO4 + 2H2O
 2Cu + O2�� + 2H2SO4��2�֣�
2Cu + O2�� + 2H2SO4��2�֣� ��2��4OH����4e��=2H2O + O2����2�֣�
��3������ձ��м���2.25gˮ��3�֣�
�����������1������Ҫ������������Ϣ���������ᵽ�Ʊ������������ɳ�ʪ��KClO3�Ͳ��ᣨH2C2O4����60��ʱ��Ӧ�Ƶõģ���˰�ʾ�÷�Ӧ��Ҫ���Ʒ�Ӧ�¶ȡ����Ƹ÷�Ӧ�¶���60�棬����ѡ��60�����ˮԡ�������ˮԡ���¶ȿ���װ�ó��ƾ��ơ��¶ȼ��⣬����Ҫ�IJ�������Ӧ������װˮԡ�����õĴ��ձ���
��2��������˵��NaClO2��Һ���õ�2�о��壬������38��ʱNaClO2������Һ������������NaClO2��3H2O������38��ʱ����������NaClO2 �����Ҫ�õ��ᾧˮ��NaClO2 ���壬Ҫ��֤�ڹ��˵�ʱ���¶ȸ���38�棬��˲��ó��ȹ��ˡ�
��3���ữ��ClO2��Һ�м���KI�������Һ������˵���еⵥ�����ɣ����Է�����������ԭ��Ӧ���ӷ���ʽΪ2ClO2 + 8H+ + 10I��="==2" Cl��+ 5I2 + 4H2O��
������֪��Ӧ����ʽ��ϵ�ã�n(ClO2)="2/5" n(I2) �� n(I2)="1/2" n(Na2S2O3)�����n(ClO2)="1/5" n(Na2S2O3)������c (ClO2 )= 1/5��c��V2 ��V1 =
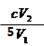 mol��L
mol��L�ӳ����¸��ձ�����ҺpH����ʱ��t�Ĺ�ϵͼ��֪���ס������ձ���ʼpHֵ����7���ס����ĵ������Һ��ǿ��ǿ���Σ����ձ���ʼpHֵС��7���������ҺΪǿ�������Ρ����ձ��ĵ缫C�������أ�����ƶ�����ԭ��Ϊ�н���Cu�������õ缫�����������ɴ˿��Եó���ԴMΪ������NΪ������a��c��eΪ������b��d��fΪ������
��1�����ձ��ĵ缫C�������أ�����ΪCu2+�ŵ磻��ҺpHֵ���ͣ�˵����Һ������OH-�ŵ磬ʹ��Һ�����������ࣻ���Ը��ݷŵ���������Ʋ�ó����еĵ������ҺΪCuSO4��Һ����˵�����Һ�Ļ�ѧ����ʽΪ2CuSO4 + 2H2O
 2Cu + O2�� + 2H2SO4 ��
2Cu + O2�� + 2H2SO4 ����2�����ձ��еĵ������ǿ��ǿ���Σ����ŵ��Ľ��У����е���ҺpHֵ���䣬������eΪH+�ŵ磬����f��OH-�ŵ磬���ʵ���ڵ��ˮ���ɴ˿��Ʋ�õ������Һ��Na2SO4�����f�缫��ӦʽΪ4OH����4e��=2H2O + O2����
��3�����ձ���c�缫����������8g����������0.125mol�ĵ���Cu����ת�Ƶ���0.25mol����˱��ձ��е������0.125mol��ˮ��Ҫʹ��Һ�ָ���Ӧ�ü���0.125mol��ˮ����2.25gˮ��

��ϰ��ϵ�д�
�����Ŀ