��Ŀ����
3�����н���ʵ������ķ�Ӧ����ʽ��ȷ���ǣ�������| A�� | ˫��ˮ�м���ϡ�����KI��Һ��H2O2+2I-+2H+�TI2+2H2O | |
| B�� | ����Ȼ�þ��Һ��������ɫ���壺2Cl-+2H2O$\frac{\underline{\;���\;}}{\;}$Cl2��+H2��+2OH- | |
| C�� | ���п��Ľ���Na¶���ڿ����У������ɹ����䰵��2Na+O2�TNa2O2 | |
| D�� | ��Ca��HCO3��2��Һ�м���������ռ���Һ�����ְ�ɫ������HCO3-+Ca2++OH-�TCaCO3��+H2O |
���� A������������ԭ��Ӧ���ɵ��ˮ��
B��������þΪ����Ӧ������ѧʽ��
C�����������ڳ��������������ƣ�
D���������ƹ�������Ӧ����̼��ơ�̼���ƺ�ˮ��
��� �⣺A��˫��ˮ�м���ϡ�����KI��Һ�����ӷ���ʽ��H2O2+2I-+2H+�TI2+2H2O����A��ȷ��
B������Ȼ�þ��Һ��������ɫ���壬���ӷ���ʽ��Mg2++2Cl-+2H2O$\frac{\underline{\;���\;}}{\;}$Cl2��+H2��+Mg��OH��2������B����
C�����п��Ľ���Na¶���ڿ����У������ɹ����䰵����ѧ����ʽ��4Na+O2�T2Na2O����C����
D����Ca��HCO3��2��Һ�м���������ռ���Һ�����ְ�ɫ���������ӷ���ʽ��2HCO3-+Ca2++2OH-�TCaCO3��+2H2O+CO32-����D����
��ѡ��A��
���� ���⿼�������ӷ���ʽ��д����ȷ��Ӧʵ���ǽ���ؼ���ע�ⷴӦ�������Է�Ӧ��Ӱ�죬��Ŀ�ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
6����ѧ����������������е���ϵ������˵������ȷ���ǣ�������
| A�� | CO2��NO2��SO2���ڴ�����Ⱦ�� | |
| B�� | ����60Co�ķ����Կ�����ijЩ������60Co��59Co��Ϊͬλ�� | |
| C�� | ������CO2���������ӻᵼ������ЧӦ�Ӿ� | |
| D�� | ʳ�ο�����ζ����Ҳ����ʳƷ������ |
11��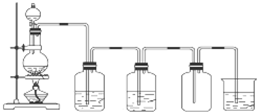 ��1��ָ��ʹ��������� ����ϴ��������Ʒ�ĵ�һ��������
��1��ָ��ʹ��������� ����ϴ��������Ʒ�ĵ�һ��������
��2���������ʵ���Ũ��Ϊ0.2mol/LNaOH��Һ500mL����ش�������⣺
��3�����в����������Ƶ�NaOH��ҺŨ��ƫ�͵���AB��
A������ʱ��NaOH����ֱ�ӷ���ֽ��
B����������NaOH����¶���ڿ�����ʱ�����
C��ѡ�õ�����ƿ��������������ˮ
D�����ձ����ܽ�NaOH��������������Һע������ƿ��
E���������ƹ���������ƿ����
��4������������Һ�������ͺ��Ȼ�����Һ ��39%���Ҵ���Һ ���Ȼ��ƺ͵������ˮ��Һ���������ϸ����Һ����ȷ���������Ƿ�Һ��������ȡ��Һ��
��5����ͼ��ʵ������ȡ������װ�ã���д������װ�������ԵIJ����������رշ�Һ©�����������ձ��м�ˮû�����ܿڣ���Բ����ƿ�������ܿ������ݣ���ֹͣ����ȴ���ܿ��γ��ȶ���ˮ������װ�õ����������ã���
 ��1��ָ��ʹ��������� ����ϴ��������Ʒ�ĵ�һ��������
��1��ָ��ʹ��������� ����ϴ��������Ʒ�ĵ�һ��������| ����-KI��ֽ�������� | ����ƿ�ռ����� | ����ƿ |
| ������ˮʪ�� | ���O��ƿ | �����Ƿ�©ˮ����©�� |
| Ӧ����NaOH������/g | Ӧѡ������ƿ�Ĺ��/mL | ������ƿ���Ҫ���������� |
| 4.0 | 500 | �ձ�����Ͳ����������ҩ�ס�������ƽ����ͷ�ι� |
A������ʱ��NaOH����ֱ�ӷ���ֽ��
B����������NaOH����¶���ڿ�����ʱ�����
C��ѡ�õ�����ƿ��������������ˮ
D�����ձ����ܽ�NaOH��������������Һע������ƿ��
E���������ƹ���������ƿ����
��4������������Һ�������ͺ��Ȼ�����Һ ��39%���Ҵ���Һ ���Ȼ��ƺ͵������ˮ��Һ���������ϸ����Һ����ȷ���������Ƿ�Һ��������ȡ��Һ��
��5����ͼ��ʵ������ȡ������װ�ã���д������װ�������ԵIJ����������رշ�Һ©�����������ձ��м�ˮû�����ܿڣ���Բ����ƿ�������ܿ������ݣ���ֹͣ����ȴ���ܿ��γ��ȶ���ˮ������װ�õ����������ã���
18��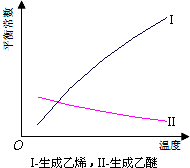 �Ҵ���ˮ��Ӧ�ڲ�ͬ�¶������µõ��IJ�����ɲ�ͬ�����dz�ѹ��ij�������������£��ֱ��Ե����Ҵ��ڲ�ͬ�¶��½�����ˮʵ���õ����ݣ�ÿ��ʵ�鷴Ӧʱ�����ͬ��
�Ҵ���ˮ��Ӧ�ڲ�ͬ�¶������µõ��IJ�����ɲ�ͬ�����dz�ѹ��ij�������������£��ֱ��Ե����Ҵ��ڲ�ͬ�¶��½�����ˮʵ���õ����ݣ�ÿ��ʵ�鷴Ӧʱ�����ͬ��
��֪���Ҵ������ѣ�CH3CH2OCH2CH3���ķе�ֱ�Ϊ78.4���34.5�森��ش��������⣺
��1���Ҵ���ˮ����ϩ�ķ�Ӧ�����ȣ�����ȡ��������ȡ�����Ӧ��������ѹǿ��ƽ������ѡ��������������������ƶ���
��2����֪��150��ʱ��1mol�Ҵ�������ˮת��Ϊ1mol��ϩ����Ӧ�ȵ���ֵΪ46KJ��д���÷�Ӧ���Ȼ�ѧ����ʽC2H5OH��g����C2H4��g��+H2O��g����H=+46KJ/mol��
��3��д���Ҵ���ˮ�����ѵķ�Ӧ��ƽ�ⳣ������ʽK=$\frac{[C{\;}_{2}H{\;}_{5}OC{\;}_{2}H{\;}_{5}]•[H{\;}_{2}O]}{[C{\;}_{2}H{\;}_{5}OH]{\;}^{2}}$�����Ҵ���ʼŨ����ͬʱ��ƽ�ⳣ��KֵԽ����ab������ĸ����
a���Ҵ���ת����Խ�� b����Ӧ���е�Խ��ȫ
c���ﵽƽ��ʱ�Ҵ���Ũ��Խ�� d����ѧ��Ӧ����Խ��
��4�����ݱ������ݷ�����150��ʱ�Ҵ�����ˮ��ȡ�����Ѳ������ڣ�ѡ����ڡ�����С�ڡ��������ڡ���125��ʱ��Ϊ���ֿ��ֶ�صõ���Ʒ���Ҵ������Ѻ��ʵķ�Ӧ�¶�������150��175�森
 �Ҵ���ˮ��Ӧ�ڲ�ͬ�¶������µõ��IJ�����ɲ�ͬ�����dz�ѹ��ij�������������£��ֱ��Ե����Ҵ��ڲ�ͬ�¶��½�����ˮʵ���õ����ݣ�ÿ��ʵ�鷴Ӧʱ�����ͬ��
�Ҵ���ˮ��Ӧ�ڲ�ͬ�¶������µõ��IJ�����ɲ�ͬ�����dz�ѹ��ij�������������£��ֱ��Ե����Ҵ��ڲ�ͬ�¶��½�����ˮʵ���õ����ݣ�ÿ��ʵ�鷴Ӧʱ�����ͬ��| �¶ȣ��棩 | �Ҵ�ת���ʣ�%�� | �л����ﺬ������������� | |
| ��ϩ��%�� | ���ѣ�%�� | ||
| 125 | 20 | 8.7 | 90.2 |
| 150 | 68 | 16.7 | 82.2 |
| 175 | 88 | 32.3 | 66.8 |
| 200 | 90 | 86.9 | 12.1 |
��1���Ҵ���ˮ����ϩ�ķ�Ӧ�����ȣ�����ȡ��������ȡ�����Ӧ��������ѹǿ��ƽ������ѡ��������������������ƶ���
��2����֪��150��ʱ��1mol�Ҵ�������ˮת��Ϊ1mol��ϩ����Ӧ�ȵ���ֵΪ46KJ��д���÷�Ӧ���Ȼ�ѧ����ʽC2H5OH��g����C2H4��g��+H2O��g����H=+46KJ/mol��
��3��д���Ҵ���ˮ�����ѵķ�Ӧ��ƽ�ⳣ������ʽK=$\frac{[C{\;}_{2}H{\;}_{5}OC{\;}_{2}H{\;}_{5}]•[H{\;}_{2}O]}{[C{\;}_{2}H{\;}_{5}OH]{\;}^{2}}$�����Ҵ���ʼŨ����ͬʱ��ƽ�ⳣ��KֵԽ����ab������ĸ����
a���Ҵ���ת����Խ�� b����Ӧ���е�Խ��ȫ
c���ﵽƽ��ʱ�Ҵ���Ũ��Խ�� d����ѧ��Ӧ����Խ��
��4�����ݱ������ݷ�����150��ʱ�Ҵ�����ˮ��ȡ�����Ѳ������ڣ�ѡ����ڡ�����С�ڡ��������ڡ���125��ʱ��Ϊ���ֿ��ֶ�صõ���Ʒ���Ҵ������Ѻ��ʵķ�Ӧ�¶�������150��175�森
8��ijѧϰС����������װ�ý���CO2�뱥��NaCO2��Һ��Ӧ�Ʊ�NaHCO3ʵ��
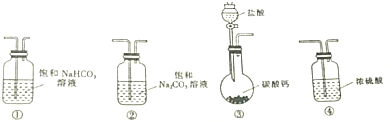
��1��ѡȡ��Ҫ��ʵ��װ�ã���ȷ������˳��Ϊ�ۢ٢ڣ�����ţ�
��2��Ϊȷ���ƵõĹ�����Ʒ�Ǵ�����NaHCO3��С��ͬѧ�������ʵ�鷽��
����������Ʒ��Һ�뱥�ͳ���ʯ��ˮ��Ӧ���۲�����
�ҷ���������Ʒ��Һ��BaCl4���۲�����
�����������pH��
�����������ط�����
��������������������������
��Ϊ�ж��ҷ����Ŀ����ԣ�ijͬѧ�÷�������NaHCO3���Ƶ���Һ����BaCl4��Һ�������Ͻ���ʵ�飬������£�
�ٴ�ʵ���ѿ�˵���ҷ����Dz����еģ������������ݣ���ͨ������˵���������ǵ�ԭ��Q=c��Ba2+����c��CO32-��=$\frac{0.2}{2}$��0.0011=1.1��10-4��5.1��10-9��
[��֪0.1mol•L-1NaHCO3��Һ�������c��CO32-��Ϊ0.0011mol•L-1��Ksp��BaCO3��=5.1��10-9]
�ڲ������ǵ����ӷ���ʽΪBa2++2HCO3-=BaCO3��+CO2��+H2O��
��ʹ��pH�ƽ��вⶨ�ı�������ȡ�������Ĺ�����Ʒ�ͷ�����NaHC03��Ʒ�ֱ��ܽ��ڵ�����ˮ�У��ֱ���pH�Ʋ�pH��
��3��ij��Һ�к���I-��Cl-�����ӣ�ȡһ������Ũ��Һ�������еμ�AgNO3��Һ����AgCl��ʼ����ʱ����Һ��$\frac{c��{I}^{-}��}{c��C{l}^{-}��}$Ϊ��4.8��10-7����֪Ksp��AgCl��=1.8��10-10��Ksp��Agl��=8.5��10-17��

��1��ѡȡ��Ҫ��ʵ��װ�ã���ȷ������˳��Ϊ�ۢ٢ڣ�����ţ�
��2��Ϊȷ���ƵõĹ�����Ʒ�Ǵ�����NaHCO3��С��ͬѧ�������ʵ�鷽��
����������Ʒ��Һ�뱥�ͳ���ʯ��ˮ��Ӧ���۲�����
�ҷ���������Ʒ��Һ��BaCl4���۲�����
�����������pH��
�����������ط�����
��������������������������
��Ϊ�ж��ҷ����Ŀ����ԣ�ijͬѧ�÷�������NaHCO3���Ƶ���Һ����BaCl4��Һ�������Ͻ���ʵ�飬������£�
| NaHCO3��Һ BaCl3Ũ�� | 0.2mol•L-1 | 0.1mol•L-1 | 0.02mol•L-1 |
| 0.2mol•L-1 | ���� | ���� | �������� |
| 0.1mol•L-1 | ���� | �������� | ������ |
| 0.02mol•L-1 | �������� | ������ | ������ |
[��֪0.1mol•L-1NaHCO3��Һ�������c��CO32-��Ϊ0.0011mol•L-1��Ksp��BaCO3��=5.1��10-9]
�ڲ������ǵ����ӷ���ʽΪBa2++2HCO3-=BaCO3��+CO2��+H2O��
��ʹ��pH�ƽ��вⶨ�ı�������ȡ�������Ĺ�����Ʒ�ͷ�����NaHC03��Ʒ�ֱ��ܽ��ڵ�����ˮ�У��ֱ���pH�Ʋ�pH��
��3��ij��Һ�к���I-��Cl-�����ӣ�ȡһ������Ũ��Һ�������еμ�AgNO3��Һ����AgCl��ʼ����ʱ����Һ��$\frac{c��{I}^{-}��}{c��C{l}^{-}��}$Ϊ��4.8��10-7����֪Ksp��AgCl��=1.8��10-10��Ksp��Agl��=8.5��10-17��
15������м�����ᡢ����������Һ��Ӧ��ȡ1molAl��OH��3��������Ҫ����HC1��NaOH�����ʵ���Ϊ��������
| A�� | 3 mol��3 mol | B�� | 1 mol��1 mol | ||
| C�� | 0.75 mol��0.75 mol | D�� | 0.25 mol��0.25 mol |
12�������й������仯���������������ȷ���ǣ�������
| A�� | �����������ֱ������������������������������Һ��Ӧ�Ƶõ����������� | |
| B�� | ���Ȼ�����Һ�еμӹ�����ˮ�����յõ�������Һ | |
| C�� | ��������������������Ũ���ᷴӦ�Ƶô�����NO2 | |
| D�� | FeCl3��Һ�������ɵõ���ˮ�Ȼ������� |
13���ס������ֹ������ʵ��ܽ��������ͼ��ʾ������˵����ȷ���ǣ�������
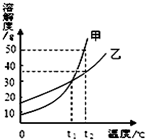

| A�� | ���ܽ�ȴ����ҵ��ܽ�� | |
| B�� | �����¶ȿ�ʹ�IJ�������Һ��Ϊ������Һ | |
| C�� | t1��ʱ���ס������ֱ�����Һ����������������� | |
| D�� | t2��ʱ��50g�Ҽ���100gˮ�еõ��ҵIJ�������Һ |
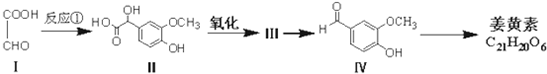
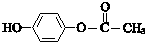 ��
��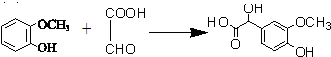 ��
��