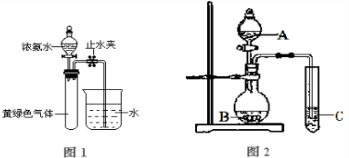
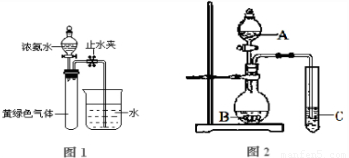
��Ŀ����
��֪Cl2ͨ��Ũ��ˮ�лᷢ�����·�Ӧ��3Cl2��8NH2![]() 6NH4Cl��N2���������Ϊ11.2L������Ϊ3.335g��Cl2��N2�Ļ������ͨ��Ũ��ˮ�������Ϊ0.672L������Cl2��N2��ռ50��(�������)]��(����������ڱ�״���²ⶨ)
6NH4Cl��N2���������Ϊ11.2L������Ϊ3.335g��Cl2��N2�Ļ������ͨ��Ũ��ˮ�������Ϊ0.672L������Cl2��N2��ռ50��(�������)]��(����������ڱ�״���²ⶨ)
(1)�����㣬�������İ�������Ϊ________g��(����д���������)
(2)��������λѧ���Ӳ�ͬ�ǶȽ������ʱ���еĵ�һ����ʽ�����ж���������δ֪��x�ֱ��ʾʲô��������д�ڱ����ڣ�(Ϊ��㣬��λδ�г�)

�𰸣�
������
������
|
����(1)0.34g ����(2)�������İ������ʵ���,ԭ���������Cl2�ڱ���µ����,ԭ��������е�Cl2��������� |

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
| |||||||||||
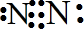
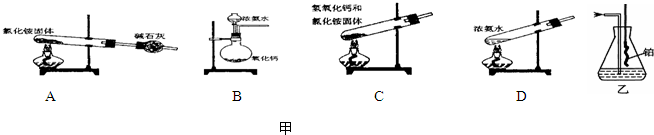 �ڰ�����ʹʪ��ĺ�ɫ��ʯ����ֽ������ԭ���÷���ʽ��ʾ��
�ڰ�����ʹʪ��ĺ�ɫ��ʯ����ֽ������ԭ���÷���ʽ��ʾ��