��Ŀ����
����Ŀ����Ȼ�����ϸ��������������F�ĺϳ�·�����£�

��֪����RCHO+R��CH2CH=CH2![]()
![]()
�� (R��R���������ԭ�ӡ������������)
(R��R���������ԭ�ӡ������������)
(1)F��һ���������ܷ���________��________��________�ȷ�Ӧ(��д3��)
(2)�Լ�a��������________��
(3)�����Լ�b������˵������ȷ��________(����ĸ���)��
a������˳���칹��b��������ˮ
c���ܷ���ȡ�����ӳɺ�������Ӧd��������NaOH��Һ������Ӧ
(4)C������Ӧ����D�ķ�Ӧ������________��
(5)B���Լ�E��Ӧ����F�Ļ�ѧ����ʽ��______________________________________________��
(6)C�Ľṹ��ʽ��______________________________��
(7)�������![]() ��
��![]() �ṹ��D������ͬ���칹����________��(������˳���칹��)��д�������������ֵĽṹ��ʽ___________________________��_________________________��
�ṹ��D������ͬ���칹����________��(������˳���칹��)��д�������������ֵĽṹ��ʽ___________________________��_________________________��
���𰸡� �Ӿ� �ӳ� ���� ��ȩ ad ������Ӧ 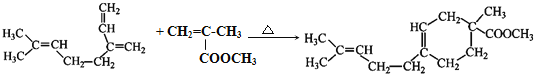
![]() 7 CH2=CHCH2COOH CH3CH=CHCOOH��CH2=CHCOOCH3��HCOOCH2CH=CH2��HCOOCH=CHCH3��CH3COOCH=CH2��HCOOC(CH3)=CH2(����������)��
7 CH2=CHCH2COOH CH3CH=CHCOOH��CH2=CHCOOCH3��HCOOCH2CH=CH2��HCOOCH=CHCH3��CH3COOCH=CH2��HCOOC(CH3)=CH2(����������)��
��������A�����Ӿ۷�Ӧ�ϳ���Ȼ������AΪCH2=C(CH3)CH=CH2���Ա� CH2=C(CH3)��A�Ľṹ�������Ϣ�ٿ�֪�Լ�aΪHCHO��A���Լ�b����ȡ����Ӧ����B���Ա�B��F�Ľṹ��֪EΪCH2=C(CH3)COOCH3��D�ͼ״�����������Ӧ����E����DΪCH2=C(CH3)COOH��CH2=C(CH3)�����õ�C��C��������Һ��Ӧ�õ�D����CΪCH2=C(CH3)CHO��
��1����F�Ľṹ��ʽ��֪��F�������������У�̼̼˫�������������Է����Ӿ۷�Ӧ���ӳɷ�Ӧ��������Ӧ��ˮ�ⷴӦ�ȣ���2��������������֪���Լ�a�Ľṹ��ʽ��HCHO������Ϊ��ȩ����3������b�Ľṹ��ʽ��֪��a��̼̼˫��������һ��������̼ԭ������2������������˳���칹�壬a����b������±������������ˮ��b��ȷ��c��b����̼̼˫�������Է����ӳɷ�Ӧ��������Ӧ��������ԭ�ӣ��ܷ���ȡ����c��ȷ��d��������ԭ�ӣ�����NaOH��Һ����ȡ����Ӧ��d����ѡad����4��CΪCH2=C(CH3)CHO��C��������Һ����������Ӧ����D����5��B���Լ�E��Ӧ����F�Ļ�ѧ����ʽ�ǣ�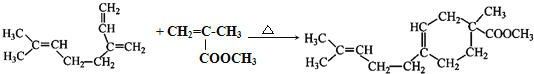 ����6��C�Ľṹ��ʽΪ��CH2=C(CH3)CHO����7��DΪCH2=C(CH3)COOH�������к���̼̼˫���������ṹ��D������ͬ���칹ΪCH2=CHCH2COOH��CH3CH=CHCOOH��CH2=CHCOOCH3��HCOOCH2CH=CH2��HCOOCH=CHCH3��CH3COOCH=CH2��HCOOC(CH3)=CH2������7�֡�
����6��C�Ľṹ��ʽΪ��CH2=C(CH3)CHO����7��DΪCH2=C(CH3)COOH�������к���̼̼˫���������ṹ��D������ͬ���칹ΪCH2=CHCH2COOH��CH3CH=CHCOOH��CH2=CHCOOCH3��HCOOCH2CH=CH2��HCOOCH=CHCH3��CH3COOCH=CH2��HCOOC(CH3)=CH2������7�֡�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ��POC13�������뵼����Ӽ������άԭ�ϣ�ʵ�����Ʊ�POC13���ⶨ��Ʒ������ʵ��������£�
I.ʵ�����Ʊ�POC13��
������������Һ̬PCl3����ȡPOC13��ʵ��װ�ã����ȼ��г������ԣ���ͼ:

���ϣ���Ag+SCN-==AgSCN��:Ksp(AgCl)>Ksp(AgSCN)��
��PCl3��POC13�������Ϣ���±���
���� | �۵�/�� | �е�/�� | ��Է������� | ���� |
PCl3 | -112.0 | 76.0 | 137.5 | �����ܣ���Ϊ��ɫҺ�壬��ˮ�����ҷ�Ӧ���ɺ�������Ȼ��� |
POC13 | 2.0 | 106.0 | 153.5 |
(1)B����ʢ���Լ���________������ܵ�������_____________________��
(2)POC13��ˮ��Ӧ�Ļ�ѧ����ʽΪ____________________________��
(3)װ��B�����ó�����O2�⣬����_____________________________��
(4)��Ӧ�¶�Ҫ������60~65�棬ԭ���ǣ�____________________________��
II.�ⶨPOC13��Ʒ�ĺ�����
ʵ�鲽�裺
���Ʊ�POC13ʵ�����������ƿ�е�Һ����ȴ�����£�ȷ��ȡ30.7g POC13��Ʒ������ʢ��60.00 mL����ˮ��ˮ��ƿ��ҡ������ȫˮ�⣬��ˮ��Һ���100. 00 mL��Һ��
��ȡ10. 00 mL��Һ����ƿ�У�����10.00 mL 3.2mol/L AgNO3����Һ��
�ۼ�����������������ҡ����ʹ�������汻�л��︲�ǡ�
����XΪָʾ������0.2 mol/L KSCN��Һ�ζ�������AgNO3��Һ���ﵽ�ζ��յ�ʱ����ȥl0.00 mLKSCN��Һ��
(5)����������������������__________________________��
(6)�������XΪ____________________ ��
(7)��Ӧ��POC13�İٷֺ���Ϊ_________��ͨ��__________(�����)������߲�Ʒ�Ĵ��ȡ�



