ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩ“―÷ΣH2S «“Μ÷÷Εΰ‘Σ»θΥαΘ§ΜΊ¥π“‘œ¬Έ ΧβΘΚ
Θ®1Θ©0.1mol/L NaHS»ή“Κœ‘Φν–‘Θ§‘ρc(S2Θ≠)___________c(H2S)Θ®ΧνΓΑ¥σ”ΎΓ± Θ§ΓΑ–Γ”ΎΓ± ΜρΓΑΒ»”ΎΓ± Θ© ΓΘ
Θ®2Θ©“―÷Σ≥ΘΈ¬œ¬Θ§CaS±ΞΚΆ»ή“Κ÷–¥φ‘ΎΤΫΚβΘΚCaS(s)![]() Ca2ΘΪ(aq)ΘΪS2Θ≠(aq)ΓΓΠΛHΘΨ0ΓΘ
Ca2ΘΪ(aq)ΘΪS2Θ≠(aq)ΓΓΠΛHΘΨ0ΓΘ
ΔΌΈ¬Ε»…ΐΗΏ ±Θ§Ksp________ (ΧνΓΑ‘ω¥σΓ±ΓΔΓΑΦθ–ΓΓ±ΜρΓΑ≤Μ±δΓ±Θ§œ¬Ά§)ΓΘ
ΔΎΒΈΦ”…ΌΝΩ≈®―ΈΥαΘ§c(Ca2ΘΪ)________Θ§‘≠“ρ «__________________________________(”ΟΈΡΉ÷ΚΆάκΉ”ΖΫ≥Χ ΫΥΒΟς)ΓΘ
Θ®3Θ©»τœρCaS–ϋΉ«“Κ÷–Φ”»κCu(NO3)2»ή“ΚΘ§…ζ≥…“Μ÷÷ΚΎ…ΪΙΧΧεΈο÷ Θ§–¥≥ωΗΟΙΐ≥Χ÷–Ζ¥”ΠΒΡάκΉ”ΖΫ≥Χ Ϋ______________ΓΘ
ΓΨ¥πΑΗΓΩ–Γ”Ύ ‘ω¥σ ‘ω¥σ Ε‘”ΎCaS(s)![]() Ca2ΘΪ(aq)ΘΪS2Θ≠(aq)ΤΫΚβΘ§Φ”»κHClΘ§‘ω¥σH+≈®Ε»Θ§H+”κS2-ΫαΚœΘ§ΫΒΒΆS2-≈®Ε»Θ§ΒΦ÷¬»ήΫβΤΫΚβ’ΐœρ“ΤΕ· CaS (s) + Cu2+ (aq) = CuS (s) + Ca2+ (aq)
Ca2ΘΪ(aq)ΘΪS2Θ≠(aq)ΤΫΚβΘ§Φ”»κHClΘ§‘ω¥σH+≈®Ε»Θ§H+”κS2-ΫαΚœΘ§ΫΒΒΆS2-≈®Ε»Θ§ΒΦ÷¬»ήΫβΤΫΚβ’ΐœρ“ΤΕ· CaS (s) + Cu2+ (aq) = CuS (s) + Ca2+ (aq)
ΓΨΫβΈωΓΩ
(1)0.1mol/L NaHS»ή“Κœ‘Φν–‘Θ§ΥΒΟςHS-Υ°Ϋβ≥ΧΕ»¥σ”ΎHS-Βγάκ≥ΧΕ»ΘΜ
(2)Ε‘”ΎCaS(s)Ca2+(aq)+S2-(aq)ΓςHΘΨ0Θ§ΈΣΈϋ»»Ιΐ≥ΧΘ§‘ρ…ΐΗΏΈ¬Ε»Θ§ΤΫΚβ’ΐœρ“ΤΕ·Θ§Φ”»κ―ΈΥαΘ§Ω……ζ≥…HS-Θ§ΤΫΚβ’ΐœρ“ΤΕ·ΘΜ
(3)œρCaS–ϋΉ«“Κ÷–Φ”»κCu(NO3)2»ή“ΚΘ§…ζ≥…“Μ÷÷ΚΎ…ΪΙΧΧεΈο÷ Θ§”Π…ζ≥…CuSΓΘ
(1)0.1mol/L NaHS»ή“Κœ‘Φν–‘Θ§ΥΒΟςHS-Υ°Ϋβ≥ΧΕ»¥σ”ΎHS-Βγάκ≥ΧΕ»Θ§Υ°Ϋβ…ζ≥…H2SΘ§Βγάκ…ζ≥…S2-Θ§‘ρc(S2-)–Γ”Ύc(H2S)ΘΜ
(2)ΔΌΕ‘”ΎCaS(s)Ca2+(aq)+S2-(aq)ΓςHΘΨ0Θ§ΈΣΈϋ»»Ιΐ≥ΧΘ§‘ρ…ΐΗΏΈ¬Ε»Θ§ΤΫΚβ’ΐœρ“ΤΕ·Θ§‘ρKsp‘ω¥σΘΜ
ΔΎΕ‘”ΎCaS(s)![]() Ca2ΘΪ(aq)ΘΪS2Θ≠(aq)ΤΫΚβΘ§Φ”»κHClΘ§‘ω¥σH+≈®Ε»Θ§H+”κS2-ΫαΚœΘ§ΫΒΒΆS2-≈®Ε»Θ§ΒΦ÷¬»ήΫβΤΫΚβ’ΐœρ“ΤΕ·Θ§ΗΤάκΉ”≈®Ε»‘ω¥σΘΜ
Ca2ΘΪ(aq)ΘΪS2Θ≠(aq)ΤΫΚβΘ§Φ”»κHClΘ§‘ω¥σH+≈®Ε»Θ§H+”κS2-ΫαΚœΘ§ΫΒΒΆS2-≈®Ε»Θ§ΒΦ÷¬»ήΫβΤΫΚβ’ΐœρ“ΤΕ·Θ§ΗΤάκΉ”≈®Ε»‘ω¥σΘΜ
(3)œρCaS–ϋΉ«“Κ÷–Φ”»κCu(NO3)2»ή“ΚΘ§…ζ≥…“Μ÷÷ΚΎ…ΪΙΧΧεΈο÷ Θ§”Π…ζ≥…CuSΘ§άκΉ”ΖΫ≥Χ ΫΈΣCaS(s)+Cu2+(aq)=CuS(s)+Ca2+(aq)ΓΘ

ΓΨΧβΡΩΓΩΡ≥ΙΛ“ΒΖœΥ°÷–÷ς“ΣΚ§”–Cr3ΘΪΘ§Ά§ ±ΜΙΚ§”–…ΌΝΩΒΡFe3ΘΪΓΔFe2ΘΪΓΔAl3ΘΪΓΔCa2ΘΪΚΆMg2ΘΪΒ»Θ§«“Υα–‘Ϋœ«ΩΓΘΈΣΜΊ ’άϊ”ΟΘ§Ά®≥Θ≤…”Ο»γœ¬Νς≥Χ¥ΠάμΘΚ
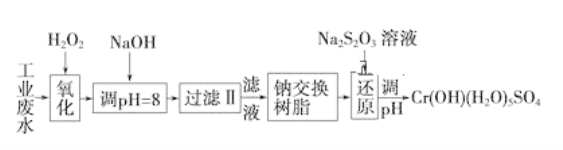
ΉΔΘΚ≤ΩΖ÷―τάκΉ”≥ΘΈ¬œ¬“‘«β―θΜ·Έο–Έ ΫΆξ»Ϊ≥ΝΒμ ±»ή“ΚΒΡpHΦϊœ¬±μΓΘ
«β―θΜ·Έο | Fe(OH)3 | Fe(OH)2 | Mg(OH)2 | Al(OH)3 | Cr(OH)3 |
pH | 3.7 | 9.6 | 11.1 | 8 | 9(>9»ήΫβ) |
Θ®1Θ©―θΜ·Ιΐ≥Χ÷–Ω…¥ζΧφH2O2Φ”»κΒΡ ‘ΦΝ «________(Χν–ρΚ≈)ΓΘ
AΘ°Na2O2 BΘ°HNO3 CΘ°FeCl3 DΘ°KMnO4
Θ®2Θ©Φ”»κNaOH»ή“ΚΒς’ϊ»ή“ΚpHΘΫ8 ±Θ§≥ΐ»ΞΒΡάκΉ” «________ΘΜ“―÷ΣΡΤάκΉ”ΫΜΜΜ ς÷§ΒΡ‘≠άμΘΚMnΘΪΘΪnNaRΓζMRnΘΪnNaΘΪΘ§¥Υ≤Ϋ≤ΌΉς±ΜΫΜΜΜ≥ΐ»ΞΒΡ‘”÷ άκΉ” «__________ΓΘ
AΘ°Fe3ΘΪBΘ°Al3ΘΪCΘ°Ca2ΘΪDΘ°Mg2ΘΪ
Θ®3Θ©ΜΙ‘≠Ιΐ≥Χ÷–Θ§ΟΩœϊΚΡ0.8 mol Cr2O72-ΉΣ“Τ4.8 mol eΘ≠Θ§ΗΟΖ¥”ΠάκΉ”ΖΫ≥Χ ΫΈΣ____________ΓΘ