��Ŀ����
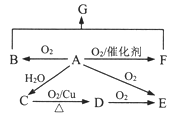
����Ŀ��ij�ǽ�������A����ͼ��ʾ�Ĺ���ת��Ϊ������D����֪DΪǿ�ᣬ��ش��������⣺
![]()
��1����A������Ϊ����ɫ���壬B���д̼�����ζ����ɫ���塣
����A��D��ˮ��Һ����ʹʪ�����ɫʯ����ֽ��죬��DΪǿ�ᣬ��A��D�ֱ�Ϊ(��д��ѧʽ) D��____________��
��д��B��C��Ӧ�Ļ�ѧ����ʽ��___________________________________________����������������������������������
��2����A�ڳ�����Ϊ���壬C�Ǻ���ɫ���塣
��A�Ļ�ѧʽ______________
��д��B��C��Ӧ�Ļ�ѧ����ʽ��______________________________
��D��Ũ��Һ�ڳ����¿���ͭ��Ӧ������C���壬�÷�Ӧ�Ļ�ѧ����ʽΪ��______________________________��
���𰸡� H2SO4 2SO2+O2![]() 2SO3 N2 2NO+O2= 2NO2 Cu+4HNO3(Ũ)=Cu(NO3)2+2NO2��+2H2O
2SO3 N2 2NO+O2= 2NO2 Cu+4HNO3(Ũ)=Cu(NO3)2+2NO2��+2H2O
���������ǽ�������A������ͼ��ʾ�Ĺ���ת��Ϊ������D��DΪǿ�ᣬB��C�����������AԪ��Ϊ�ɱ��Ԫ�أ�����������Ԫ����S��NԪ�أ���A�ǵ��������ʡ�
��1����A�ڳ�����Ϊ����ɫ���壬��A��S���ʣ�B����ɫ�д̼�����ζ����ɫ���壬��B��SO2����������������������������C��SO3�����������ˮ��Ӧ�������ᣬ��D��H2SO4��
�ٸ�������������D��H2SO4����SO2ת��Ϊ��������ķ���ʽΪ2SO2+O2![]() 2SO3��
2SO3��
��2����A�ڳ�����Ϊ���壬C�Ǻ���ɫ���壬��A��N2��C��NO2��B��NO��D��HNO3����
�ٸ�������������AΪN2����NOת��ΪNO2�ķ���ʽΪ 2NO+O2=2NO2���۳����£�ͭ��Ũ���ᷴӦ��������ͭ��NO2��ˮ����Ӧ�Ļ�ѧ����ʽΪCu+4HNO3(Ũ)=Cu(NO3)2+2NO2��+2H2O��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�