��Ŀ����
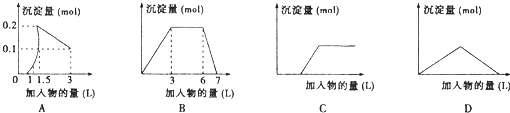
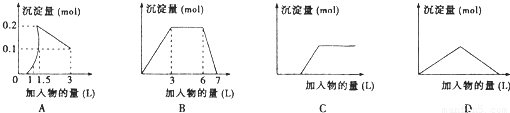
��������ͼ��������Ϊ����������������Ϊ��������������Ӧѡ���ȷ���ǣ� ��
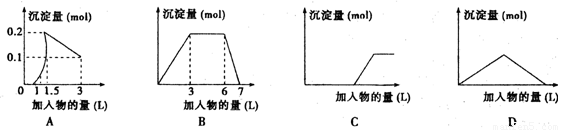
A����1LŨ�Ⱦ�Ϊ0.1mol/L��Ba(OH)2��NaAlO2���Һ�м���0.1 mol/LϡH2SO4��Һ
B������0.1 mol/L AlCl3��0.3mol/L NH4Cl��1L���Һ�м���0.1mol/LNaOH��Һ
C�����ռ���Һ�еμ�������Һ
D����Ba(OH)2��Һ����ͨ�������̼
���𰸡�
A
��������

��ϰ��ϵ�д�
�����Ŀ
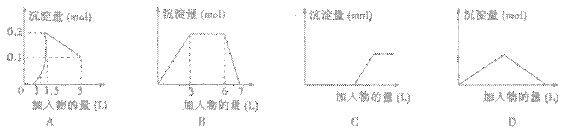
��������ͼ��������Ϊ����������������Ϊ���������������ȷ���ǣ�������
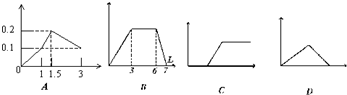

| A����1LŨ�Ⱦ�Ϊ0.1mol/L��Ba��OH��2��KAlO2���Һ����0.1mol/LϡH2SO4 | B����0.1mol/L ������0.3mol/L NH4Cl�Ļ��Һ1L�м���0.1mol/L NaOH��Һ | C��������������Һ�μӹ�����ˮ | D����Ca��OH��2��Һ����ͨ�������̼ |