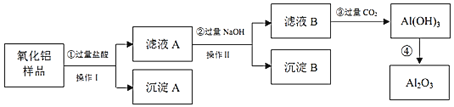
��Ŀ����
����Ŀ��һ��������������(CH3CH2CH2CH2CH3)���������ѽⷴӦ��
��.CH3CH2CH2 CH2CH3 (g)CH3CH===CH2(g)��CH3 CH3 (g) ��H1����274.2 kJ��mol��1
��.CH3CH2CH2CH2CH3(g)CH3CH2CH3(g)��CH2===CH2(g) ��H2����122.7 kJ��mol -1
�ش��������⣺
��1���ں��º�ѹ���ܱ������У�����һ�����������鷢���ѽⷴӦ����ʼʱ�������Ϊ a L��һ��ʱ�䷴Ӧ�ﵽƽ������������Ϊ b L����ʱ�������ת���� ��(������)��_________����Ӧ��ϵ�г���һ������ˮ����(ˮ �����ڸ������²����뷴Ӧ)���ٴ�ƽ����������ת���ʽ�_____(��������������С������������)��ԭ��Ϊ_______________��
��2���¶�Ϊ T ��ʱ����ѹǿ��Ϊ 100 kPa ���ܱ������г��� 1 mol��L��1 CH3CH=CH2�� 2 mol��L��1 CH3CH3������Ӧ��CH3CH===CH2(g)��CH3CH3(g) CH3CH2CH3(g)��CH2===CH2(g)��H3����� CH3CH2CH3 �����ʵ���Ũ����ʱ�� t �ı仯��ͼ����������ʾ��
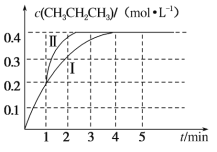
����H3��_____��
�ڸ÷�Ӧ��ƽ�ⳣ�� Kp��_____��(Kp Ϊ�Է�ѹ��ʾ��ƽ�ⳣ������ѹ����ѹ�����ʵ��������������� ���� 2 λС��)��
������ 1 min ʱ���ı�ijһ��Ӧ��������������Ϊ����������ı������Ϊ_____��
��3���� 0.1 mol CH3CH3��ȫȼ�պ������ͨ�� 100 mL 3 mol��L��1�� NaOH ��Һ�У���ַ�Ӧ��������Һ������Ũ�ȵĴ�С˳��Ϊ_____��
��4����ϡ����Ϊ�������Һ��CH3CH3 ȼ�ϵ�صĸ�����ӦʽΪ_____��
���𰸡�![]() ���� ����ˮ������������������൱�ڼ�ѹ������ƽ�������ƶ� -151.5 kJ��mol��1 0.17 �����Ч���� c��Na+����c��HCO3-����c��CO32-����c��OH-����c(H+)
���� ����ˮ������������������൱�ڼ�ѹ������ƽ�������ƶ� -151.5 kJ��mol��1 0.17 �����Ч���� c��Na+����c��HCO3-����c��CO32-����c��OH-����c(H+) ![]()
��������
��1�����������鷢���������ѽⷴӦ�ķ���ʽ��1L�������ѽ�����2L���壬��1L��������ȫ�ѽ⣬�������1L����Ӧ��ϵ�г���һ������ˮ��������������൱�ڼ�ѹ��
��2���ٸ��ݸ�˹���ɼ���CH3CH==CH2(g)��CH3CH3(g)![]() CH3CH2CH3(g)��CH2==CH2(g)���ʱ䣻
CH3CH2CH3(g)��CH2==CH2(g)���ʱ䣻
������������ʽ������ƽ�ⳣ����
��1 min ʱ���ı�ijһ��Ӧ��������Ӧ���ʼӿ죬ƽ��û���ƶ���
��3��0.1 mol CH3CH3��ȫȼ������0.2mol������̼���壬ͨ�� 100 mL 3 mol��L��1�� NaOH ��Һ�У�����̼Ԫ�ء���Ԫ���غ㣬��֪����0.1mol Na2CO3��0.1 mol NaHCO3��
��4����ϡ����Ϊ�������Һ�� CH3CH3�ڸ���ʧ�������ɶ�����̼���塣
��1�����������鷢���������ѽⷴӦ�ķ���ʽ��1 L�������ѽ�������2 L���壬��1L�������ѽ⣬�������1 L�����Ϊ a L��������ﵽƽ������������Ϊ b L���������(b-a)L����ֽ������������Ϊ(b-a)L���������ת���� ��(������)��![]() ����Ӧ��ϵ�г���һ������ˮ��������������൱�ڼ�ѹ��ƽ�������ƶ����������ת��������
����Ӧ��ϵ�г���һ������ˮ��������������൱�ڼ�ѹ��ƽ�������ƶ����������ת��������
��2������.CH3CH2CH2 CH2CH3 (g)![]() CH3CH=CH2(g)��CH3 CH3 (g) ��H1����274.2 kJ��mol��1��
CH3CH=CH2(g)��CH3 CH3 (g) ��H1����274.2 kJ��mol��1��
��.CH3CH2CH2CH2CH3(g)![]() CH3CH2CH3(g)��CH2=CH2(g) ��H2����122.7 kJ��mol -1��
CH3CH2CH3(g)��CH2=CH2(g) ��H2����122.7 kJ��mol -1��
���ݸ�˹������������CH3CH=CH2(g)��CH3CH3(g)![]() CH3CH2CH3(g)��CH2=CH2(g) ��H3=��122.7 kJ��mol -1��274.2 kJ��mol��1= ��151.5 kJ��mol��1��
CH3CH2CH3(g)��CH2=CH2(g) ��H3=��122.7 kJ��mol -1��274.2 kJ��mol��1= ��151.5 kJ��mol��1��
��
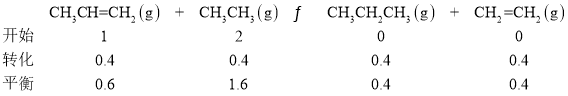
Kp��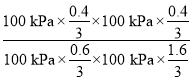 ==0.17
==0.17
��1 min ʱ���ı�ijһ��Ӧ��������Ӧ���ʼӿ죬ƽ��û���ƶ������Ըı�������Ǽ����Ч������
��3��0.1 mol CH3CH3��ȫȼ������0.2 mol CO2���壬ͨ�� 100 mL 3 mol��L��1�� NaOH ��Һ�У�����̼Ԫ�ء���Ԫ���غ㣬��֪����0.1 mol Na2CO3��0.1mol NaHCO3��̼����ˮ��̶ȴ���̼�����ƣ�������Һ������Ũ�ȵĴ�С˳��Ϊc��Na+����c��HCO3-����c��CO32-����c��OH-����c(H+)��
��4����ϡ����Ϊ�������Һ�� CH3CH3�ڸ���ʧ�������ɶ�����̼���壬������Ӧʽ��![]() ��
��

 ��ѧ��ʦ����ϵ�д�
��ѧ��ʦ����ϵ�д�