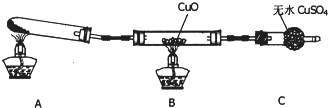
��Ŀ����
ij�о���ѧϰС���ѧ����һ�λ�У���Ƴ����·������ⶨͭ�����ԭ��������

ָ����ʦ��Ϊ�˷������У��ṩ��һƿ����һ����H2SO4��Fe2(SO4)3���ʵ�CuSO4��Һ���������ҩƷ�����ġ�
(1)ͬѧ���������۲�����CuSO4��Һ�Ƿ���Ҫ�ᴿ����������ѧϰС���ѧ�����������Ŀ�����(�����������ֻ��ѡ��a��b��С����һ��������һ�ʲ��ػش�)
a����Ҫ�ᴿ������________(��д�Լ���ѧʽ)����Ӧ���˼����ᴿCuSO4��Һ��
b�������ᴿ��������
________________________________________________________________________��
(2)��ͭ��ʯī�����缫���CuSO4��Һʱ��ͭ�缫Ӧ�ӵ�Դ��________����ʯī�缫�ϵĵ缫��Ӧʽ��________________________________________����һ���ķ�Ӧʽ��
________________________________________________________________________��
(3)��ʵ����ͭ�����ԭ��������
________________________________________________________________________
(�ú�m��V�Ĵ���ʽ��ʾ)��
(1)a.CuO[��Cu(OH)2��CuCO3]
(2)����4OH����4e��=2H2O��O2����Cu2����2e��=Cu
(3) 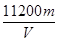
��������
���������(1)���ʻ�Ӱ��CuSO4��Һ�ĵ�⣬��ΪFe3���������Ա�Cu2��ǿ�����ʱ�����ȷŵ磬���Ա����ᴿ�����Լ���CuO��Cu(OH)2��CuCO3�����ʣ�������Һ������ԣ�ʹFe3����Fe(OH)3����ʽ�������������˳�ȥ��
(2)��Ϊ�����ϱ�����Cu2���ŵ磬����ֻͭ����Ϊ������ֻ�����Դ�ĸ���������ʯī��������OH���ŵ硣
(3)���ݵ�ⷴӦ����ʽ2CuSO4��2H2O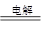 2Cu��2H2SO4��O2����֪�����ɵ�ͭ�����ʵ��������������ʵ���֮����2:1����
2Cu��2H2SO4��O2����֪�����ɵ�ͭ�����ʵ��������������ʵ���֮����2:1���� �����M(Cu)��
�����M(Cu)��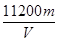 ��
��
���㣺�������ʵ��ᴿ���缫���ƺ͵�������жϡ��缫��Ӧʽ����д�Լ����ԭ�������ļ���
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣�����Ե������ͭ�ⶨͭԭ�ӵ����ԭ������Ϊ���壬ּ�ڿ���ѧ��������õ��ԭ�����ʵ�����������������������ѧ���������������淶�Ͻ���ʵ���������������ѧ����ѧ��������

 ������ÿ�ʱ�Ż���ҵϵ�д�
������ÿ�ʱ�Ż���ҵϵ�д�